Implants
MedCAD Titanium Facial Reconstruction Plates Now 3D Printed
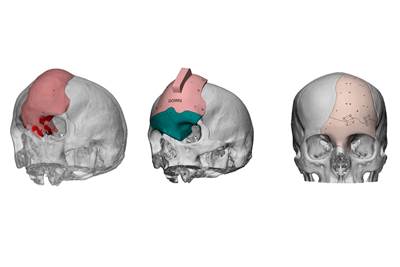
MedCAD, creators of AccuPlate patient-matched titanium implants, has announced that AccuPlate 3DTi reconstruction plates for the mandible and midface are now 3D printed.
Read More3D Printed Metal Vibenite 350 Testing Indicates Biocompatibility for Implant Use
The material is both wear and corrosive resistant, and could be well suited as an implant material where wear resistance is essential, such as human joints.
Read MoreCranial Implant 3D Printed From Hydroxyapatite Ceramic: The Cool Parts Show #76
Cranial implants are typically made from titanium or PEEK; in this episode of The Cool Parts Show, we look at how implants made from a bioceramic can improve osseointegration and healing.
Watch3D Printed Spine Implants Made From PEEK Now in Production
Medical device manufacturer Curiteva is producing two families of spinal implants using a proprietary process for 3D printing porous polyether ether ketone (PEEK).
Read MoreFDA Approves 3D Systems’ Orthopedic Patient-Specific Guides for Ankle Replacements
The Total Ankle Patient-Matched Guides pair with Smith+Nephew’s Total Ankle replacement solutions and are designed to help surgeons save time and ensure accuracy while performing fewer steps than standard instrumentation.
Read MoreColibrium Additive Develops Spectra M EB-PBF Printer for Smaller Build Volumes
The printer offers a smaller build volume making it well suited for a variety of additive manufacturing users, including medical and orthopedic implant manufacturers looking to further reduce cost-per-part and additive production costs.
Read MoreResearchers Develop Resin for 3D Printing Implants for Cataracts, Other Eye Conditions
The ability to 3D print intraocular devices for treating cataracts and other eye conditions could significantly enhance eye care for patients by offering unparalleled levels of customization and design precision, potentially leading to better clinical outcomes.
Read More3D Printed PEEK Spine Implants in Production: The Cool Parts Show Bonus
Curiteva is using Fused Strand Deposition to produce two different lines of FDA-cleared spine implants. We visited the company’s Huntsville, Alabama, facility to learn more.
WatchArcomedLab Utilizes Biomedical 3D Printing for 700 Successful Craniomaxilofacial Implants in Latin America
The company specializes in biomedical 3D printing with a focus on patient-centric solutions.
Read More3D Systems Receives FDA Clearance for World’s First 3D Printed PEEK Cranial Implants
The FDA clearance now enables widespread adoption of 3D Systems’ self-contained, cleanroom environment-based printing system, the EXT 220 MED, with medical-grade PEEK materials to deliver patient-specific cranial reconstruction solutions.
Read More