ArcomedLab Utilizes Biomedical 3D Printing for 700 Successful Craniomaxilofacial Implants in Latin America
The company specializes in biomedical 3D printing with a focus on patient-centric solutions.
Share
Read Next
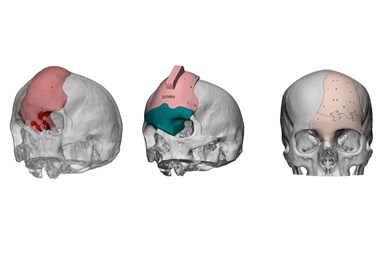
ArcomedLab, a Chilean-North American company specializing in biomedical 3D printing, says it has achieved the world’s largest case list for craniomaxillofacial implants. The company counts 700 successful cases across Latin America, including Chile, Peru, Colombia and Mexico, as it aims to transform the health care industry with cutting-edge additive manufacturing (AM) technology and expertise.
“For us it is an honor to lead the biomedical 3D printing sector in the region, with a case list of patients that makes us extremely proud, and with PEEK and Titanium 3D printing technology that is unique in Latin America,” says Ilan Rosenberg, ArcomedLab CEO and co-founder.
The company says the implants have very unique advantages because they are printed in polyether ether ketone (PEEK), which can mean that porosities and geometries are replicated perfectly. Also, the material can interact with soft tissues such as muscle and skin, and there are no distortions in radiographic examinations. PEEK is compatible with irradiation of cancer patients and offers bioactivation, if needed. The material is biologically inert and biomechanically similar to human bone. It can be easily removed and put in place again if complications arise. And it is a sustainable option that generates less waste.
At the heart of ArcomedLab’s success is its state-of-the-art facility in Chile, featuring a dedicated clean room for 3D printing implants and personalized medical devices in a highly controlled sterile environment. The company says this unique infrastructure enables ArcomedLab to deliver high-quality, custom-made solutions that meet the specific needs of patients and health care providers.
Looking ahead, ArcomedLab aims to expand its presence throughout Latin America, forging partnerships with leading clinical groups and universities in the region. By combining expertise, technology and strategic vision, ArcomedLab aims to stay at the forefront of biomedical 3D printing innovation, driving positive change in the MedTech landscape.
With that aim, the company has created the ArcomedLab Institute to serve as a platform for collaboration with the academic world, offering professional training and access to the specialized biomedical 3D printing laboratory. The company also recently appointed Daniel Martínez to the position of Innovation Strategy and Growth manager. He has experience in the medtech, innovation and clinical sectors, offering insights and strategic vision to drive ArcomedLab’s growth strategy in the Latin America and U.S. markets.
“I am excited to join ArcomedLab and contribute to advancing biomedical 3D printing in the orthopedic area, on upper and lower extremities as well as spine personalized solutions,” Martínez says. “Our second goal is to leverage the ArcomedLab Institute’s resources and know-how to further develop innovative academic programs in the field of biomedical 3D printing in alliance with top universities and medical schools, to accelerate the introduction of these technologies in clinical settings and improve patient outcomes with cost-effective and personalized solutions.”
Related Content
-
FDA Approves 3D Systems’ Orthopedic Patient-Specific Guides for Ankle Replacements
The Total Ankle Patient-Matched Guides pair with Smith+Nephew’s Total Ankle replacement solutions and are designed to help surgeons save time and ensure accuracy while performing fewer steps than standard instrumentation.
-
PrinterPrezz/Vertex Manufacturing Rebrands to Zeda
The rebranding is said to reflect the company’s “Z to A” approach, which means it starts first with the customer in mind and ends with a product.
-
Production AM Demands Process and Procedures — More Machines Will Come Later
Arch Additive has transitioned to full production of implants made through electron beam melting. The transition has involved practices and personnel, not equipment. As customer products win approval and go to market, here are 5 operational moves the AM implant manufacturer has made.