FDA Grants Formlabs Emergency Use Authorization for 3D Printed Ventilator Component
Formlabs has the capacity to produce up to 3,000 3D printed adapters a day and is now shipping the adapters to hospitals for critically ill COVID-19 patients.

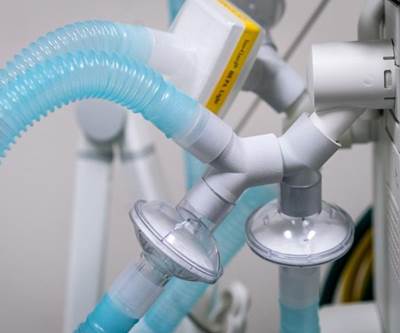
3D printed T piece adaptor. Credit: Northwell Health.
This article originally appeared on the website of Plastics Technology, sister publication to Additive Manufacturing.
Formlabs has received emergency use authorization (EUA) from the U.S. Food and Drug Administration (FDA) to print bi-level positive airway pressure (BiPAP) adapters designed by Northwell Health, New York's largest healthcare provider. Formlabs is reportedly the first 3D printing manufacturer to receive an EUA and is now shipping these adapters to hospital systems throughout the U.S. to combat the shortage of ventilators and provide life-saving treatment to COVID-19 patients.
The 3D printed adapters convert BiPAP machines, typically used for patients suffering from sleep apnea, into functional invasive mechanical ventilators. The key component to converting the BiPAP machine is a small, plastic T-shape adapter, which is produced on 3D printing machines in hospitals across the country. Formlabs will also produce these adapters at its FDA-registered headquarters in Somerville, MA to distribute to hospitals and government systems throughout the United States.
Formlabs will allocate 150 3D printers at its headquarters towards printing these adapters, enabling the company to print up to 3,000 parts per day once production is fully ramped up.
“Prior to the COVID-19 pandemic, the FDA had only authorized a handful of EUAs over a 30 year period,” said Max Lobovsky, CEO and co-founder of Formlabs. “Formlabs’ EUA for BiPAP adapters signifies the need for these components and 3D printings' unique ability to fill that need. 3D printing enables rapid iteration and prototyping of new, innovative medical equipment, while expediting the production process, shortening supply chains, and allowing for localized manufacturing. Hospitals around the country can also use Formlabs’ printers to create these adapters locally under their own practice of medicine, meaning printing the adapters at scale in the hardest-hit areas is as easy as uploading a design and pressing print.”
Northwell Health has been a key partner of Formlabs throughout the COVID-19 pandemic. The hospital system began working with Formlabs in 2018 and that partnership has led to the creation of 3D printed test swabs, in collaboration with the University of South Florida Health, and now 3D printed BiPAP adapters. Northwell designed these adapters to provide life-saving care to hospitalized COVID-19 patients during the peak of the virus in New York City.
After using these adapters on a number of patients in their New York City ICUs during the peak of the virus in the city, Formlabs will now print these adapters at scale to aid hospitals in other hard-hit cities.
Back in March, I talked with Dávid Lakatos, Formlabs' Chief Product Officer and he mentioned how the company was looking to work with the medical community:
“Formlabs is looking for ways to help the medical community address the overall COVID-19 epidemic. We have many customers in the healthcare space already using Formlabs products for COVID-19 related projects, and recently launched the Formlabs Support Network for COVID-19 Response. This is an initiative to match Formlabs customers who are willing to use their printers to support healthcare needs with projects that could use their assistance.”
For more information on Formlabs response to COVID-19 visit, formlabs.com/covid-19-response. Those looking to volunteer their time and 3D printers to help combat COVID-19 can find more information here.
Related Content
8 Cool Parts From Formnext 2023: The Cool Parts Show #65
New additive manufacturing technologies on display at Formnext were in many cases producing notable end-use components. Here are some of the coolest parts we found at this year’s show.
Read More3D Printed PEEK Spine Implants in Production: The Cool Parts Show Bonus
Curiteva is using Fused Strand Deposition to produce two different lines of FDA-cleared spine implants. We visited the company’s Huntsville, Alabama, facility to learn more.
Read MoreUnderstanding PEKK and PEEK for 3D Printing: The Cool Parts Show Bonus
Both materials offer properties desirable for medical implants, among other applications. In this bonus episode, hear more from Oxford Performance Materials and Curiteva about how these companies are applying PEKK and PEEK, respectively.
Read MoreZeda AM Production Plant in Ohio Now Open — Thoughts on the New Facility
73,000-square-foot metal powder bed fusion plant includes extensive machining capability plus separate operational models for serving medical versus other businesses.
Read MoreRead Next
3D Printed Device Allows Multiple Patients on One Ventilator
Emergency authorization from FDA allows for use of device permitting four patients to use a shared ventilator. HP involved in mass production via additive manufacturing.
Read MoreVentilator Manufacturing: Challenges and Likely Response During Coronavirus Crisis
Can we achieve something like a wartime transition to ventilator production, or will the response need to look different from this?
Read More3D Printing and Coronavirus: U.S. Additive Manufacturers Share Their Experiences
The COVID-19 outbreak has brought both setbacks and opportunities for American manufacturing. 3D printing companies share their stories.
Read More