3D Printing and Coronavirus: U.S. Additive Manufacturers Share Their Experiences
The COVID-19 outbreak has brought both setbacks and opportunities for American manufacturing. 3D printing companies share their stories.
Share
Read Next
Editors’ note: The editors of Additive Manufacturing have heard from 3D printing businesses in the United States that have seen both positive and negative effects from the global COVID-19 pandemic. The stories range from increased orders as OEMs look to replace parts previously sourced from Asia, to new questions about social distancing and workplace policies. We are collecting these stories and will update this page with the newest information at the top as responses come in. If you have a story to share, email us.
For more information on business conditions, see Gardner Intelligence. To find 3D printing services, see our Supplier Directory. For guidance on approaching coronavirus as an employer, we recommend the CDC website.
(Responses may be edited for length/clarity.)
May 19, 2020
Universities, Manufacturers Share COVID-19 Responses in Panel Discussion
Representatives from Phoenix Analysis & Design Testing (PADT), L3Harris, Arizona State University, the University of Colorado and Stratasys participated in a panel discussion on Thursday, May 14, 2020, titled “Fighting COVID-19 with 3D Printing.” Participants discussed the advantages of AM for filling supply chain gaps; regulatory issues around 3D printing; the importance of personal networks; and distributed collaboration coupled with distributed manufacturing. A recording of the webinar can be viewed here.
May 18, 2020
Custom Glasses Manufacturer Pivots to PPE
Fitz Frames, a manufacturer of custom glasses featuring 3D printed frames, has pivoted to produce a new line of protective eyewear called Fitz Protect. This PPE item is custom made for each customer and can incorporate prescription lenses to reduce the amount of gear needed by healthcare professionals. Watch this quarantine edition episode of The Cool Parts Show to learn more.
3D Printed Tooling to Deliver Thousands of Face Shield Headbands
In another instance of PPE production shifting away from direct 3D printing, Catalysis Additive Tooling has produced a 3D printed injection mold that is being used to produce headbands for face shields. The metal tool went from design to production in about 2 weeks. The company says it will be able to produce more than 50,000 headbands within a 3-week timeframe. —Stephanie Hendrixson, Senior Editor, Additive Manufacturing
May 13, 2020
Tech Company Hobbyists Respond to PPE Shortage
Dell Technologies posted a piece on how some of its employees are using their personal 3D printers to make personal protective equipment (PPE). The company quoted us for perspective on where this effort fits in. Here is the article.
Additive Manufacturing of Implants is Surging
3D printed medical implant maker Tangible Solutions is now producing at its highest rate yet, indirectly because of the crisis. Surgeries using these implants are currently being delayed, but will be performed as soon as they are allowed again. Implant suppliers are stocking up to get ready. Learn more in this episode of The Cool Parts Show — and be sure to subscribe to keep watching. This episode is one of a special series we’ve just produced about the ways additive manufacturing is advancing during this time. —Peter Zelinski, Editor in Chief, Additive Manufacturing
America Makes Announces Top Designs for Masks Challenge; Issues New PPE Figures
America Makes has announced the top designs for its Fit to face—Mask Design challenge. Participants were tasked with optimizing a face mask’s fit-to-face contact for a range of face types using datasets provided by NIOSH. Designs were evaluated by Autodesk and ASME; winning designs are listed here and can be found on the America Makes and NIH 3D Print Exchange websites.
America Makes also reports that its PPE repository has now matched more than 178,000 pieces of personal protective equipment from 470 manufacturers. 101 design entries have been submitted for review by the VA, NIH and FDA. —Stephanie Hendrixson, Senior Editor, Additive Manufacturing
April 30, 2020
PAPR Hoods Made Universal with 3D Printed Adapters
Superfeet and sister company Flowbuilt Manufacturing are producing powered air-purifying respirator (PAPR) hoods for hospitals in the northwestern United States. The companies, which typically produce ergonomic and custom footwear, pivoted their manufacturing capacity to produce 42,000 hoods in April and plan to deliver another 30,000 in May.
The hoods feature 3D printed adapters made on Flowbuilt’s HP Multi Jet Fusion (MJF) 3D printers. While the hoods were designed to be natively compatible with just one respirator model, these adapters allow them to be used with four of the major systems from various manufacturers. The Superfeet/Flowbuilt hoods can be sanitized using wipes or UV light, and are a reusable replacement for standard single-use hoods.
120,000+ Pieces of PPE Delivered Through America Makes Repository
America Makes has released figures for its COVID-19 repository that seeks to match the healthcare industry with suitable manufacturing capacity for PPE and other necessary items. As of April 29, the organization says that more than 120,000 pieces of PPE have been matched across the country including 91,389 non-N95 masks and 27,573 face shields. 463 manufacturers have listed their capabilities through the platform. —Stephanie Hendrixson, Senior Editor, Additive Manufacturing
April 27, 2020
3D Printing Hands the Baton to Injection Molding for Mask Adapters
Masks On, a recently-formed organization that is converting full-face snorkel masks into personal protective equipment (PPE) for healthcare providers treating COVID-19 patients, used 3D printing for prototyping and manufacturing an initial batch of 400 plastic adapters that hold medical-grade filters. The masks are classified as face shields by the FDA, and are intended for use when FDA-approved PPE is not available. For reasons related to materials and scale, the group has now turned to injection molding for making these adapters.
“We started out doing deposition 3D printing, and graduated pretty quickly to resin-based printing for a couple of different reasons. One is that it’s really difficult to sterilize deposition prints — there are too many nooks and crannies. We worked with a team at Formlabs to choose the correct resins, and went through more than eight iterations of the 3D printed parts and actually shipped a few hundred of the 3D printed devices.
Ultimately we went through a collection of tests to emulate 2, 3, 8, 12 months of use and found some degradation issues over time. I really hope people aren’t using these in three months, but that’s the gap we’re trying to bridge. We’re now working with Protolabs on injection molding the parts. Several days ago, I had 14,000 injection molded parts sitting on my porch.
Where 3D printing was really useful was in moving through those variants very quickly, so we could get things into the hands of physicians and get their feedback before committing to injection molding. But one of the joys of going through this has been watching the baton be handed between teams. It started out with a couple of really talented physicians, and then it moved over to a bunch of engineers like me, who were sort of scrambling to build something safe to the best of our ability. And then the last step is that we teamed up with a manufacturing facility and they're running faster than we ever could in terms of getting them built and out into the hands of clinicians. Each of those transitions has been really, really wonderful to watch seeing different skills and different approaches work together.” —Sanjay Vakil, Executive Director, MasksOn.org
April 22, 2020
Ventilator Splitters: “We hope you never have to use it.”
A group of four San Antonio anesthesiologists have released an open source design for a ventilator splitter and flow limiters to allow hospitals to support up to four patients on a single ventilator. According to the ventsplitter.org website, the concept of ventilator splitting dates back to a study published in 2006 where a simulation demonstrated that four patients could share a ventilator circuit using T-tubes and adapters under emergency circumstances; the technique was successfully demonstrated in practice during the 2017 Las Vegas shooting incident. The open source splitter design is intended to be a substitute for hospitals that may not have T-tubes or other parts readily available.
The Y-shaped splitters and cup-shaped flow limiters were developed for printing with fused deposition modeling (FDM) printers, though they can be made with other technologies. 3D Systems is exploring 3D printing these parts with its modular Figure 4 system, based on digital light processing (DLP). The company says it can print 2 splitters in 90 minutes from its biocompatible and sterilizable MED-AMB/SG resin, and can leverage several ISO 13485-certified facilities to make them.
While splitters (and other 3D printed devices) can be effective in an emergency situation, the caveat on the organization’s website speaks volumes: “We hope you never have to use it.” The ventilator splitter and flow limiter designs are not FDA-approved and have not been tested on humans. The devices are intended only as a last resort, and if used, should be supported with appropriate inline filters and one-way valves to prevent cross-contamination. —Stephanie Hendrixson, Senior Editor, Additive Manufacturing
April 20, 2020
Clinical Testing of 3D Printed Swabs Now Underway
Four 3D printed test swab prototypes are now being used in a clinical trial conducted by a multidisciplinary team from Beth Israel Deaconess Medical Center (BIDMC), according to an article posted on the Center’s website. The 3D printed designs are being used in concordance with standard nasopharyngeal (NP) test swabs on patients arriving at a drive-thru testing site to compare the results. So far, three of the four prototypes have completed this testing with performance on par with the conventional swabs.
This clinical test marks the third and final stage in BIDMC’s effort to develop and identify alternative swabs suitable for COVID-19 testing. The four prototypes were chosen from a field of more than 100 designs submitted by organizations including Carbon and Resolution Medical; Envisiontec; Formlabs; HP; OPT Industries; Origin; Stanford University; the University of South Florida; and the University of Washington. A preprint of the group’s work is available at medrxiv.org. —Stephanie Hendrixson, Senior Editor, Additive Manufacturing
April 16, 2020
Compliance Standards Higher for Production-Scale PPE
The latest 3D Printing and Coronavirus Check-In video discusses more about the shift to scale production in addressing medical equipment needs. Extol Inc. shared this thought:
“One other angle regarding production-scale additive manufacturing of PPE: The bar for compliance to FDA and medical industry certifications seems to have been held lower for the makers who have been donating much of what they have made and have been working at a smaller scale. As production-scale additive grows, it seems like the regulatory/certification requirements become more of a hurdle. It may be that because production additive can now meet a different scale of demand, it receives more scrutiny.” —Kyle Harvey, Director of Marketing, Extol Inc.
The Fight for PPE Is About More than Quantities
In our first conversation directly with a hospital representative regarding the coronavirus pandemic, the TriHealth hospital system of Cincinnati, Ohio, shared some of its efforts to provide adequate personal protective equipment (PPE) to its staff. Director of Lean Mike Waterman emphasized that rather than raw quantities, he and his colleagues are looking for solutions that are safely reusable. One that is now in place: A 3D printed hard hat adapter made by GE Additive that converts any hard hat into a face shield and can be sterilized using the system’s existing equipment. —Stephanie Hendrixson, Senior Editor, Additive Manufacturing
April 14, 2020
Ramping Up to 1 Million Testing Swabs Per Week
Resolution Medical is a Carbon Production Network partner that has stepped up to process thousands of 3D printed test swabs used to diagnose COVID-19. Carbon and its partners are part of the PrintedSwabs.org consortium along with Formlabs, Envisiontec, and Origin.
“The swab was an open source effort out of Beth Israel Deaconess Medical Center (BIDMC) and Harvard Medical School. They were able to evaluate almost 150 3D printed designs and narrowed it down to a few leading ones and started testing. These swabs need to be packaged at an FDA-registered medical device company — and that’s where we came in. Carbon contacted us and we took the lead on the project from there. We’ve been able to get all the testing done and register the device with the FDA. There should be results from formal clinical studies coming out soon, and we’re selling swabs now. Basically over the span of a month, we’ve been able to turn a printed swab into a medical device.
We’re printing some swabs at Resolution Medical because we have the six Carbon printers. Then we’re also sourcing swabs from dental labs that have been converted to making these. Right now we’re focused on getting more and more dental labs and internal printing labs. We do a validation process for each new lab that involves design testing and physical testing, and then we do ongoing inspection like we do with any other supplier. So it’s inspection, then packaging and labeling. Right now we are manually packaging each swab individually, but we have automated packaging systems coming in. We’re ramping up, and by the end of this week we should be at 1 million a week coming through Resolution.” —Shawn Patterson, Founder and President, Resolution Medical
Face Shield Nesting Strategy Improves Productivity
Avid Product Development has employed its design for additive manufacturing expertise and capacity to devise and produce a face shield frame optimized for 3D printing with HP Multi Jet Fusion (MJF) technology. The DFAM face shield prints in a compact geometry for maximum efficiency, allowing for approximately 290 parts in a full build or 145 parts in a half build. Avid is offering the design for free to help other members of the digital manufacturing community to deliver critical personal protection equipment to health care workers. The design can be downloaded at avidpd.com.
Weekly Check-in: The Next Big Production Story Is Swabs
In this week’s video, Peter Zelinski and I recap a number of coronavirus-related production stories, including one where 3D printing has been used to make mold tooling rather than the parts themselves. We discuss masks and face shields (including a new face shield solution that attaches to a hard hat), as well as the next big production story for additive manufacturing: testing swabs. A coalition of manufacturers are now working together to provide 3D printed swabs, which can be ordered at printedswabs.org. Watch the video here. —Stephanie Hendrixson, Senior Editor, Additive Manufacturing
April 8, 2020
Appeal for Data: What AM Materials Are Safe for Use Near the Face in 3D Printed Masks?
The FDA has approved a 3D printed mask design — the first medical mask to receive this approval as part of the coronavirus response. In a related appeal on Twitter, Dr. Beth Ripley, senior innovation fellow with the Veterans Health Administration Innovation Ecosystem (developer of the mask), sought additive manufacturing materials expertise. She wrote:
“3D printing community: I need your help. We are trying to evaluate multiple 3D printed face mask designs for non-hospital use. Here is our concern: What if the plastics that are used in FDM and SLA printing are releasing volatile organic compounds (VOCs)? Especially concerning as this is on the face.
“Is this really ‘#BetterThanABandana’? Does anyone have good data to communicate relative risks of breathing through common FDM/PLA materials such as PLA, PETG and others? Powder bed nylon parts seem to be relatively safe, but if you have data to the contrary, let me know.”
Early responses affirmed the view that nylon is safe material in this regard, and included a link to 3D Systems chart of material properties for COVID-19 applications.
For latest responses, find the Twitter thread here.
April 7, 2020
Weekly Check-In: A Pivot Toward Production
In this week’s coronavirus and 3D printing discussion video, Peter Zelinski and I talk about a recent shift we’ve seen: Emergency 3D printing and maker movements giving way to production-level 3D printing of PPE and bridge production of ventilator components. On the horizon? Possibly a return to conventional manufacturing for many of these items as the supply chain begins to catch up. We also discuss needs for PPE beyond the hospital, 3D printed test swabs and more. Watch here.
3DHeals Webinar Addresses 3D Printing’s Usefulness in Pandemic
Over the weekend 3DHEALS, an organization that promotes the use of 3D printing in healthcare, hosted a virtual meeting of a group of medical professionals and manufacturing experts to discuss the coronavirus situation. Representatives from FluidForm, HP, Origin, PrinterPrezz, the National Additive Manufacturing Innovation Cluster (NAMIC) and more discussed how their organizations are utilizing 3D printing and answered questions from the audience. The group covered 3D printing projects underway such as face shields, masks and swabs; discussed ventilator splitters, traceability and FDA guidance; and weighed in on where 3D printing can be best deployed. The full conversation is archived here and well worth a watch. —Stephanie Hendrixson, Senior Editor, Additive Manufacturing
April 6, 2020
Cautions and Promise for 3D Printed PPE
Rapid Application Group of Tulsa, Oklahoma is finalizing a 3D printed mask combining SLS printed nylon with N-95 or HEPA filter material; the item is intended for use by first responders and medical support staff, and has been developed in cooperation with the VA and FDA.
“Field-level or nonsurgical PPE can save lives right now, and maybe put some of those other N-95 masks and other surgically approved items into the specific areas where they are needed. We spent a lot of time planning to make sure we get this right. These are medical devices so you have to be careful; you can’t make any assumptions with this kind of scenario.” —Jason Dickman, Co-Founder and Chief Operating Officer
“Once everybody hears about 3D printing PPE, it sounds like a natural fit. We’ve seen all of these companies and home makers jump on the bandwagon of printing a mask… we want to make sure this is correct — that the material science is good, and the design is good.
But the awesome thing about additive manufacturing is that it’s so agile. Within 48 hours we went from learning of the COVID-19 problem with masks, into design, into fit check. It’s showing that we can actually solve real-world problems utilizing additive manufacturing. This is no longer a novelty. We are now saving lives with 3D printing.” —Terry Hill, Co-Founder and CEO
April 1, 2020
Long-Term Implications of Supply Chain “Friction,” and Getting Beyond Direct 3D Printing
There’s a little more friction between the links along the global supply chain today, and there’s going to be more pressure to look for simplified supply chain solutions. 3D printing is an option for companies that might need tooling but don’t want to go the traditional route of shipping from overseas.
We have an expedited project right now related to the COVID response because of the PPE shortage. We still have a skeleton crew operating the lab, printing tools with the intent to allow us to quickly manufacture parts that will help relieve the shortage. We’re looking for a route to help transition from printing discrete parts — which is great early on, but then you start to hit that asymptote and can’t quite meet the demand that we’re starting to see across the States. We can be a catalyst to take those designs and spin them up in traditional forms of manufacturing to make tens of hundreds of thousands very quickly. —Josh Martin, Co-Founder and CEO, 3DFortify
March 31, 2020
Weekly Check-In: More Face Shields, Updates on Masks and Movement from the FDA
In our second video conversation, editor-in-chief Peter Zelinski and I take a look at new developments for 3D printing in the fight against COVID-19. Its usage for PPE has only grown, with concerted efforts by 3D printer suppliers and large manufacturers to produce face shields and more. At least one new design for a reusable N-95 mask has come to light. And, we’re seeing emergency use authorizations from the FDA, a sign that innovative 3D printed devices are welcome — but also an indication of the seriousness of the crisis. Watch the discussion. —Stephanie Hendrixson, Senior Editor, Additive Manufacturing
March 30, 2020
America Makes Opens Matchmaking Repository, Pathway to FDA Qualification
In a webinar held earlier today, America Makes executive director John Wilczynski said that the organization aims to be a “matchmaker” for additive manufacturers and healthcare providers. The group is collecting information from both parties with the goal of connecting hospitals with manufacturers who can help meet their needs.
"We're in a perfect position to bring the community together around a challenge. We can convene, coordinate and catalyze a community to address a specific problem,” Wilczysnki said.
The group’s COVID-19 activities also include a way to submit designs for 3D printed devices. Designs are being evaluated for viability by the VA, and can enter directly into a pathway for clinical use or FDA approval if required. —Stephanie Hendrixson, Senior Editor, Additive Manufacturing
March 27, 2020
FDA Publishes FAQs on 3D Printing for Medical Devices, PPE
The FDA has released guidance in response to increased interest in 3D printed medical devices, masks, ventilators and other items to support coronavirus treatment. “While the FDA understands that 3D printing may occur to provide wider availability of devices during the COVID-19 public health emergency, some devices are more amenable to 3D printing than others,” the post reads. Manufacturers and facilities are invited to contact the Administration for more information about 3D printing specific items. —Stephanie Hendrixson, Senior Editor, Additive Manufacturing
March 26, 2020
FDA Issues Emergency Use Authorization for 3D Printed Ventilator Component
Prisma Health in South Carolina announced that it has received emergency use authorization from the U.S. Food and Drug Administration (FDA) for a ventilator expansion device allowing a single ventilator to support up to four patients at once during times of acute ventilator shortages such as those being seen and anticipated with the COVID-19 crisis. More on the new 3D printed VESper device. —Peter Zelinski, Editor in Chief, Additive Manufacturing
3D Printed Parts May Be More Expensive, But Help Keep Companies in Business
For companies who have gone overseas, their parts could be six to 12 months out. To recreate a conventional tool you’re talking about investing maybe $35,000 to $40,000. But in the interim, I can 3D print those parts from the same sort of materials that they’re using. It’s obviously a little more expensive, maybe $8 or $10 per part that might have previously cost 35 cents. But at least now, they can be supplied with parts. You’re either going to have a zero margin and lose money, or use this as a handicap to get people parts that they need in a few days. At least you’re getting product out, and you’re helping your staff. Once all this clears out, then you can start to ramp up and you have the capital to stay in business. —Jared Crooks, Founder, A.M. ToolBox LLC
March 25, 2020
Video Conversation Addresses Ventilators, PPE, Business Conditions
With so much news about coronavirus and 3D printing’s potential to help, Editor-in-Chief Peter Zelinski and I filmed a conversation this week to compare notes and try to put the news in context. We expect that this will be a recurring exercise, for as long as it makes sense.
You can find the video here. In this installment, we discuss ventilator parts, personal protective equipment (PPE) like respirators and face shields, and coronavirus’s impact on business conditions. —Stephanie Hendrixson, Senior Editor, Additive Manufacturing
Situation Is “Increasing Opportunities” for Service Bureaus, AM Contractors
With the situation, we are seeing a slow up in machine sale activities. Most states are mandating only essential employees can work on-site, so that's bound to have an impact. But, I see that as short term only. The good thing is it's increasing opportunities with all the service bureaus I am involved with. And those who have positioned themselves to be additive manufacturing contract manufacturers are in a very good place right now. It’s opening doors and windows for us that didn’t used to exist from the service side of things. I think it’s going to be good for additive. —Ginger Ruddy, Senior Account Executive | Business Development, Cimquest
March 24, 2020
Tooling May Be a Better Solution Than Direct 3D Printing
In this coronavirus crisis, we are getting a lot of requests from thermoforming and plastic injection molding companies for our 3D printed tools. In some cases we are getting requests for mass production-level parts. Many of the needs right now are tens of millions of parts — direct 3D printing is possible, but not at these high volumes.
Traditional metal tools are capable of supplying high volumes of parts, but take weeks or months to produce. Because of the quick manufacturing and turnaround time, additive manufactured tooling is a perfect fit to the support the current situation we are all in. Catalysis FX tooling [made with sand binder jetting] is ideal for thermoforming of parts; 3D printed metal injection mold tools have a very fast turnaround time and should be considered for plastic injection molding. Additive manufactured tooling can be produced much faster than a traditional manufactured tool, and is an excellent bridge to supply parts quickly. —Rick Shibko, Director of Business Development, Catalysis Additive Tooling
March 23, 2020
Medical Community Works to Receive AM’s Response to Equipment Shortage
An addendum to the item below. I had the chance to join a conference call today involving hospitals, medical device makers and AM technology providers that addressed working with the manufacturing community at large to meet the looming emergency shortage of medical supplies. Making parts answers only part of the challenge. People on the call with me described the efforts underway to address other challenges, including:
- Sterilization of reusable face shields such as those cited below. High-temperature sterilization might damage the shield; other approaches are being explored.
- Quality control for incoming protective gear produced in many different places, to assure a wearer is not put at risk because of a product defect.
- Logistics of receiving gear from many different sources, and distributing it to different hospitals in the right proportions.
The latter two items are potentially related. Organizations such as Amazon and FEMA have been approached as potentially being able to organize this part of the manufacturing response. —PZ
3D Printers Aid in Shortage of Medical Protective Equipment
News is coming to us of 3D printing companies dedicating their efforts and capacity toward producing protective equipment for health care workers. The leader of Massachusetts General Hospital put out a call for exactly this help. Stratasys is working to address needs such as this, using its own capacity as well as drawing on the efforts of additive manufacturing companies volunteering to get involved. A husband-and-wife maker of custom 3D printers, Budmen Industries, is now working to produce 300 protective face shields in the couple's basement. —Peter Zelinski, Editor-in-Chief, Additive Manufacturing
March 20, 2020
Can Manufacturers Help with Ventilators? Stay Tuned
Perhaps the most frequent question I am receiving right now from manufacturers: How can we get involved with ventilator production? How can we help? The simple answer for now is: I don’t know. As I described in this post on the ventilator shortage, just part-making capacity by itself likely isn’t the answer to the need or hope to produce ventilators quickly. Crowdsourcing of ventilator parts may or may not represent a viable portion of the answer. However, our other aim in posting and circulating that item on ventilators is to communicate that we reach a community of manufacturers, and that (to some extent, at least) we understand the nuances of the need involved. We will keep posting and circulating items along these lines as one of the ways to make clear, to anyone close to the ventilator problem who might be searching, that we can facilitate connections to manufacturers if and when ventilator makers see and define the need where outsourced or crowdsourced manufacturing capacity can help.
In response to one recent social media post on ventilators, manufacturer and 3D printing user Hummingbird Scientific posted this video on ventilators. As the Hummingbird president noted, a “non-trivial” piece of equipment. —Peter Zelinski, Editor-in-Chief, Additive Manufacturing
Supplier Map Visualizes 3D Printing Service Providers
In previous post I wrote about U.S. additive manufacturers who have been asked to make parts and tooling as a result of supply chain disruptions. These companies are serving customers that have experienced slowdowns in getting needed components first from Asia, and now potentially other parts of the world.
The response to that post led me to take our existing directory of 3D printing service providers and visualize it into a public Google Map. If you are a business in need of a mold, end-use part, prototype, spare or replacement part to keep production running, take a look at the 3D printing service providers near you. One of them may be able to help.
(If you provide 3D printing services, you can add your company with this Google Form. I will import new data into the map as frequently as I can.) —Stephanie Hendrixson, Senior Editor, Additive Manufacturing
3D Printing Service Bureaus Can Help “Resolve Supply Chain Disruptions”
We understand manufacturing is being affected by the outbreak of the coronavirus, as it impacts supply chains and causes interruption in operations. Using a 3D printing service bureau based in the U.S. can help resolve supply chain disruptions and enables manufacturers to keep producing their parts and products. Not only can we print production quantities at Avid, but we can leverage our in-house mechanical engineering team to reverse engineer and design parts and products for additive manufacturing. —Doug Collins, Co-owner, Avid Product Development
March 18, 2020
On the Post-Pandemic Horizon: Distributed and Additive Manufacturing
I think the future is more distributed manufacturing. Companies like Xometry and others can enable U.S. companies to tap into manufacturers all across the country. In the past, that visibility just wasn't available to customers. They just didn't know there was a great machine shop in North Dakota or in Utah, and that they have the ability not only to tap into those skills, but also to mitigate supply chain risks that rise up in these kind of situations. I think the increase use of additive and other advanced manufacturing will also tip the scales more towards a skilled workforce and there'll be less focus on the low-cost work, and so that will also help the United States. The percentage of your cost base to produce something will be less about the the hourly wage of the employee and more about equipment, skill and output.
The constant improvements of additive manufacturing technology and the trend towards mass customization will also cause more of a focus on smaller manufacturers because you won't need somebody who can make a million parts. Sometimes you need somebody who can chunk out five or ten thousand. That also plays really well for U.S. manufacturing.
I wouldn't say we have seen any widespread changes over the past two weeks in terms of of what is coming in on the additive side, (although) we've had a lot of inbound interest about printing respirator parts. I think the problem is that there are regulatory and other requirements that we need to work out for additive for some of these things. And it probably hasn't happened to a degree it should have for this emergency. I think this there will be a lot more work on that in the future. We've got a lot of inquiries about it. —Randy Altschuler, CEO, Xometry
3D Printed Tooling, Automation Help Footwear Production Continue
As a domestic footwear 3D printing and injection molding manufacturer, Flowbuilt has experienced less dependence on foreign producers during this time, translating into less disruption in our business. If anything, brands are looking for new solutions which may offset the risk they see in their overseas supply chains. We have experienced a steady stream of inquiries and new projects beginning shortly after the aggressive tariffs were enacted last year. This has carried over into 2020 and we expect it to continue throughout the year.
Through collaboration projects with our brand partners we’ve driven innovation in the footwear creation process utilizing our HP Multi Jet Fusion (MJF) printers [for prototyping as well as] creating 3D printed injection tooling parts which is usually a CNC process on solid blocks of aluminum. The footwear industry is ripe for disruption in this area, and it feels like this is one of the innovations that will stick even after the current crisis.
Fortunately, our parent company Superfeet has implemented some smart remote work policies early in the situation which we have adopted. Besides cutting our face-to-face business development efforts during this time, we’ve reduced onsite staff to essential project-level personnel only. One of the benefits of a highly automated facility is that we can run the production with fewer bodies with less contact. Most other business operations can effectively be handled through remote connections. —Chuck Sanson, Director of Business Development, Flowbuilt Manufacturing
March 17, 2020
3D Printing Crowdsourcing to Help Overcome Supply Chain Shortages
In a public document titled “3D Printer Crowdsourcing for COVID-19,” more than 200 people from across the world have offered to help to produce vital hospital supplies amid the evolving COVID-19 outbreak. The idea came when a Northern Italian hospital needed a replacement valve for a reanimation device and the supplier had run out with no way to get more in a short time. An Italian company brought a 3D printer directly to the hospital and redesigned and then produced the missing piece.
I think this is a great idea to help hospitals care for an expected influx of coronavirus patients in times where there might be an inadequate supply of critical hospital equipment. This encouraging initiative of additive manufacturers shows their willingness to help, donate their manufacturing or design capacities in times of uncertainty — and the crucial role global networks can play in a global crisis. —Barbara Schulz, European Correspondent, Additive Manufacturing
3D Printing for Applications “That Do Not Typically Look to AM”
With the recent interruption to the supply chain due to the coronavirus, we’ve received requests to manufacture products that companies simply cannot get right now from China. We understand that a number of these customers have adopted “just in time” manufacturing processes and are carrying reduced inventory levels. While “just in time” manufacturing helps to reduce inventory and costs when manufacturing is running smoothly, it causes an immediate issue when products cannot be shipped or received.
Many of the requests we have received due to recent supply chain issues are for applications that do not typically look to additive manufacturing for design, production capacity or cost reasons. However, additive manufacturing solves their short-term product issue and allows them to keep or get product back on the shelves. —Ken Burns, VP Commercial, Forecast3D
March 16, 2020
Additive a Solution Where Standards, Material Supply Can Allow It
I heard from a senior manager with a company that supplies technology to additive manufacturing users. Off the record, he noted some of what he has been seeing with industrial 3D printing service providers in recent weeks. Many are seeing increases in business. He believes in some cases certifications or standards that would normally have ruled out additive manufacturing are being reevaluated for the sake of getting parts. One challenge with delivering on this new business: Additive manufacturing in some cases might have a supply chain extending to regions that have been affected, because raw material might come from China even though the part is printed in the States.
The business news for 3D printing in this time might actually amount to a net shift toward additive manufacturing for production as opposed to 3D printing’s more established uses. My source notes that while there are unexpected production opportunities arising from the virus situation, many larger companies are also making cuts to non-essential expenses during this time. Those cuts reduce the budget for prototyping. —Peter Zelinski, Editor-in-Chief, Additive Manufacturing
3D Printing Aids “Bridge Production of Necessities”
We have seen an increase in business from particular sectors that are suffering from supply shortage due to the slowdowns in Asia. We have also been working to aid in the bridge production of necessities such as face masks, which historically were manufactured with more traditional processes even though we have the capacity to produce them at 1,000 per day.
But, we have simultaneously seen a pausing of development and production of entirely new products. —Gabe Bentz, CEO, Slant3D
Service Bureaus “Ready and Willing to Help”
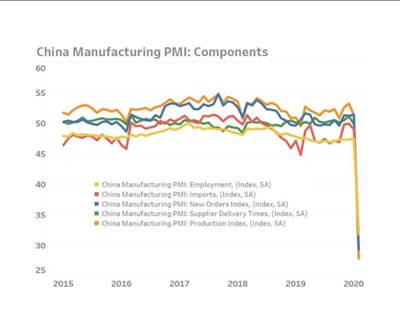
About two weeks ago I sat down with economist Michael Guckes to film a video conversation about China’s production manager’s index (PMI) and ways that U.S. companies might look to 3D printing as a solution for supply chain disruptions. Since that story posted, I’ve heard from a handful of 3D printing service bureaus with open capacity, ready and willing to help. (If this describes you, reach out to be listed in our supplier directory.)
While the situation is serious and bound to get worse before it gets better, I’m encouraged by the ingenuity and can-do attitudes of additive manufacturers. The silver lining could be that more eyes are opened to the possibilities of 3D printing as a result of the challenges U.S. manufacturing is currently facing. —Stephanie Hendrixson, Senior Editor, Additive Manufacturing
Related Content
3D Printed Titanium Replaces Aluminum for Unmanned Aircraft Wing Splice: The Cool Parts Show #72
Rapid Plasma Deposition produces the near-net-shape preform for a newly designed wing splice for remotely piloted aircraft from General Atomics. The Cool Parts Show visits Norsk Titanium, where this part is made.
Read MoreAt General Atomics, Do Unmanned Aerial Systems Reveal the Future of Aircraft Manufacturing?
The maker of the Predator and SkyGuardian remote aircraft can implement additive manufacturing more rapidly and widely than the makers of other types of planes. The role of 3D printing in current and future UAS components hints at how far AM can go to save cost and time in aircraft production and design.
Read MoreHow Machining Makes AM Successful for Innovative 3D Manufacturing
Connections between metal 3D printing and CNC machining serve the Indiana manufacturer in many ways. One connection is customer conversations that resemble a machining job shop. Here is a look at a small company that has advanced quickly to become a thriving additive manufacturing part producer.
Read MoreBeehive Industries Is Going Big on Small-Scale Engines Made Through Additive Manufacturing
Backed by decades of experience in both aviation and additive, the company is now laser-focused on a single goal: developing, proving and scaling production of engines providing 5,000 lbs of thrust or less.
Read MoreRead Next
In Coronavirus Uncertainty, 3D Printing Is a Potential Solution for U.S. Manufacturing
The impact of the COVID-19 virus has reached U.S. manufacturers by way of the supply chain. Here are several ways 3D printing could help, plus a video discussion of the data with economist Michael Guckes.
Read MoreVentilator Manufacturing: Challenges and Likely Response During Coronavirus Crisis
Can we achieve something like a wartime transition to ventilator production, or will the response need to look different from this?
Read More3D Printing Crowdsourcing to Help Hospitals Experiencing Supply Shortages Linked to COVID-19
As the virus continues to spread worldwide and breaks supply chains, 3D printers and expertise in additive manufacturing can lend a helping hand. Potential sources identify themselves using an online directory.
Read More
.jpg;width=70;height=70;mode=crop)