Health Canada Approves First Medical Implant 3D-Printed By Canadian Manufacturer
The 3D Specifit mandibular plate, patient-specific device will be used for mandibular reconstruction of patients with oral cancer.
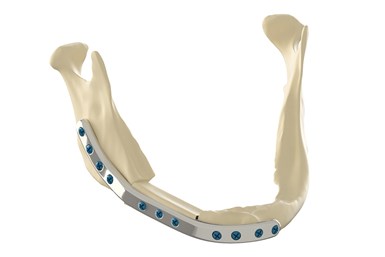
Health Canada approves first 3D-printed medical implant printed by a Canadian organization. Photo Credit: Investissement Québec – CRIQ
CHU de Québec-Université Laval and Investissement Québec – CRIQ have announced Health Canada’s approval of the first 3D-printed medical implant by the 3D anatomical reconstruction laboratory (LARA 3D) at Investissement Québec – CRIQ’s facilities in Quebec City, Canada. This is the first time a Canadian organization has been authorized to produce a 3D-printed implantable medical device in Canada. The 3D Specifit mandibular plate, patient-specific device, will be used for mandibular reconstruction of patients with oral cancer.
Launched last year, LARA 3D has been ISO 13485 certified since April 2021, which confirms that its manufacturing process for new medical devices meets the most rigorous quality management standards. With Health Canada’s approval issued in September, the green light has been given to treat patients and produce the 3D Specifit mandibular plate along with surgical cutting and drilling guides.
3D printing or additive manufacturing (AM) is revolutionizing the manufacturing of medical implants, the company says. Surgeons are no longer limited to adapting a patient’s physiology to prostheses with predetermined dimensions. Instead, patient-specific implants made before surgery, designed from the patient’s internal imaging, follow the unique contours of the bone to be repaired. LARA 3D uses biocompatible metals for 3D printing by laser and electron beam powder bed fusion.
The company says the use of patient-specific metal prostheses that are printed instead of traditionally manufactured will improve the quality of health care in Quebec by reducing patients’ surgery and recovery times, hence improving their quality of life.
The approval of the first 3D Specifit product signals the recognition of Quebec’s expertise in medical 3D printing. Other innovations are in the pipeline in partnership with the private sector, which will benefit from the knowledge and facilities available at LARA 3D. Nearly $8 million in investments were required to construct the facilities and pay for the equipment and human resources needed for R&D and the certification process.
Related Content
-
Overcoming Challenges with 3D Printing Nitinol (and Other Oxygen-Sensitive Alloys) Through Atmospheric Control
3D printed nitinol has potential applications in dental, medical and more but oxygen pickup can make this material challenging to process. Linde shares how atmospheric monitoring and the use of special gas mixtures can help maintain the correct atmosphere for printing this shape alloy and other metals.
-
Understanding PEKK and PEEK for 3D Printing: The Cool Parts Show Bonus
Both materials offer properties desirable for medical implants, among other applications. In this bonus episode, hear more from Oxford Performance Materials and Curiteva about how these companies are applying PEKK and PEEK, respectively.
-
Durable, Waterproof 3D Printed Casts: The Cool Parts Show #58
Recovering from an injury with an ActivArmor cast means that patients can exercise, bathe and live life while they heal. We get a firsthand look at the solution in this episode of The Cool Parts Show.














