Lithoz Offers Improved Ceramic Material for 3D-Printed Bone Replacements
The company says the 3D printed, patient-specific parts made of hydroxyapatite are well suited for bone replacements in the medical and dental field.
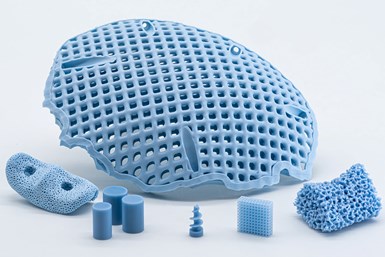
LithaBone HA 480 is a greatly improved human bone-like ceramic material which can be used to create distinctly blue complex parts. Photo Credit: Lithoz
Lithoz has developed the LithaBone HA 480, a substantially improved bioresorbable ceramic bone graft substitute. The company says the material was developed in intensive collaboration with users of previous Lithoz bone graft substitutes.
The 3D printed patient-individual parts made of hydroxyapatite are well suited for bone replacements in the medical and dental field. The company says substantial improvements include mechanical properties, higher wall thickness, possible geometries, size of the lattice structures and processability.
The development goal was to make the material more economically viable and the use of 3D printing for bone replacements even more effective. The company says the result is a greatly improved human bone-like ceramic material which can be used to create distinctly blue complex parts.
In medicine, ceramics are considered well suited materials for devices and implants due to their biocompatibility, low thermal conductivity and lighter intrinsic weight. Most importantly, however, certain ceramics can be resorbed by the body, eliminating the risks connected to second surgeries.
The wide range of bone structures in the body necessitate that ceramic materials for bone replacement fully cover this range and are both biocompatible and resorbable. The planned interaction of the bone substitute material with adjacent bone and tissues depends on the optimized design of geometries which, accordingly, determines clinical success as bone regrows along the material’s guide structures and the ceramic dissolves in the body.
LithaBone HA 480 offers several major improvements, including significantly higher wall thicknesses, which is a key requirement for versatility reasons. With LithaBone HA 480, previous maximum wall thicknesses of <1.6mm (LithaBone HA 400) have been increased to 10 mm, while minimum wall thicknesses required have been reduced even further, which significantly broadens the range of applications.
The material also enables more precise imaging accuracy of fine structures — in line with the strengths of lithography-based ceramics manufacturing (LCM). One clear goal was to offer a higher level of ultraprecise resolutions and highly complex structures when working with hydroxyapatite. It is said LithaBone HA 480 shows strongly reduced overpolymerization with a simultaneously improved depth of cure, resulting in a simpler selection of build parameters and a significantly more stable manufacturing process.
The material also offers a 10-fold longer shelf life, which helps eliminate the previous obstacle of previous materials’ short shelf life that impacted the availability required for urgent surgical interventions. Parts are also significantly easier to clean, making up valuable time during often vital medical interventions. According to the company, experienced users have confirmed significantly faster and easier cleaning processes thanks to an improved binder composition.
- Lithoz also developed the Lithoz CeraFab S65 ceramic 3D printer that features plug-and-play scalability which can drive complex, 3D-printed high-performance ceramic applications to industrial levels.
- With the acquisition of CerAMing and its LSD printing technology, Lithoz says it now fully covers all strategically relevant market potentials in ceramic 3D printing.
- Lithoz’s CeraMax Vario V900 3D printer can produce large ceramic components with thick walls and full densities using oxide and non-oxide ceramics.
- Read Peter Zelinski’s take on the some of the most important developments seen at Formnext 2022, including 3D printing on Lithoz’s photopolymer-based platform which enabled production of a ceramic pin at over 400 pieces per build.
Related Content
New Zeda Additive Manufacturing Factory in Ohio Will Serve Medical, Military and Aerospace Production
Site providing laser powder bed fusion as well as machining and other postprocessing will open in late 2023, and will employ over 100. Chief technology officer Greg Morris sees economic and personnel advantages of serving different markets from a single AM facility.
Read More3D Printed Spine Implants Made From PEEK Now in Production
Medical device manufacturer Curiteva is producing two families of spinal implants using a proprietary process for 3D printing porous polyether ether ketone (PEEK).
Read MoreUltra-Complex 3D Printed Scaffolds Enable Cell Growth: The Cool Parts Show #70
Perhaps the ultimate surface-area challenge is in bioengineering: creating structures that can grow sufficient cells within a compact volume to be effective for leading-edge medical treatments. The Southwest Research Institute develops bioreactor scaffolds that could only be made using 3D printing.
Read More3D Printed PEEK Spine Implants in Production: The Cool Parts Show Bonus
Curiteva is using Fused Strand Deposition to produce two different lines of FDA-cleared spine implants. We visited the company’s Huntsville, Alabama, facility to learn more.
Read MoreRead Next
Crushable Lattices: The Lightweight Structures That Will Protect an Interplanetary Payload
NASA uses laser powder bed fusion plus chemical etching to create the lattice forms engineered to keep Mars rocks safe during a crash landing on Earth.
Read MoreAlquist 3D Looks Toward a Carbon-Sequestering Future with 3D Printed Infrastructure
The Colorado startup aims to reduce the carbon footprint of new buildings, homes and city infrastructure with robotic 3D printing and a specialized geopolymer material.
Read MorePostprocessing Steps and Costs for Metal 3D Printing
When your metal part is done 3D printing, you just pull it out of the machine and start using it, right? Not exactly.
Read More