Scroll to unlock this premium content if video does not play.
One of the next frontiers in medicine is to treat diseases not through chemistry but through biology, using cells engineered to fight the disease. But it takes a lot of cells to accomplish this, and therefore a lot of surface area for the cells to grow on. Industrializing bioengineering demands space-efficient means to cultivate cells, entailing a surface-area challenge even greater than, say, the surface-area needs of improved heat exchangers. One organization at the forefront of this work is the Southwest Research Institute (SwRI), which has developed a bioreactor employing a geometrically complex cell-growth scaffold only possible through 3D printing. The geometry is so complex, the computer processing challenges of modeling the digital file pose an obstacle to scaling up the cell-growth hardware. A segment of this episode with Metafold discusses implicit modeling as a potential alternative for mathematically defining the most complex 3D printed geometries.| This episode of The Cool Parts Show is sponsored by Carpenter Additive
The Cool Parts Show is a video series from Additive Manufacturing Media that explores the what, how and why of unusual 3D printed parts. Watch more here.
Have a cool part to share? Email us.
Related Resources
- The Southwest Research Institute
- An introduction to implicit modeling, the approach used in software from Metafold
- On YouTube, our video playlist of geometrically complex 3D printed heat exchangers
- Newly added: A viewer of the episode shared this paper about an open-source biofabrication platform for tissue research. The “bIUreactor” developed at Indiana University (IU) takes cells like those that might be grown in the SwRI bioreactor, and uses them to make 3D tissue structures for research. The platform can be made by any lab with a 3D printer, based on downloadable files made available by the paper’s authors.
Transcript
Stephanie Hendrixson
Today on the show, we're going to be talking about bioreactors, these chambers that allow you to manage a biological process inside of a closed system. And specifically we're looking at 3D printed scaffolds that are designed to go inside of bioreactors like this for growing cells.
Peter Zelinski
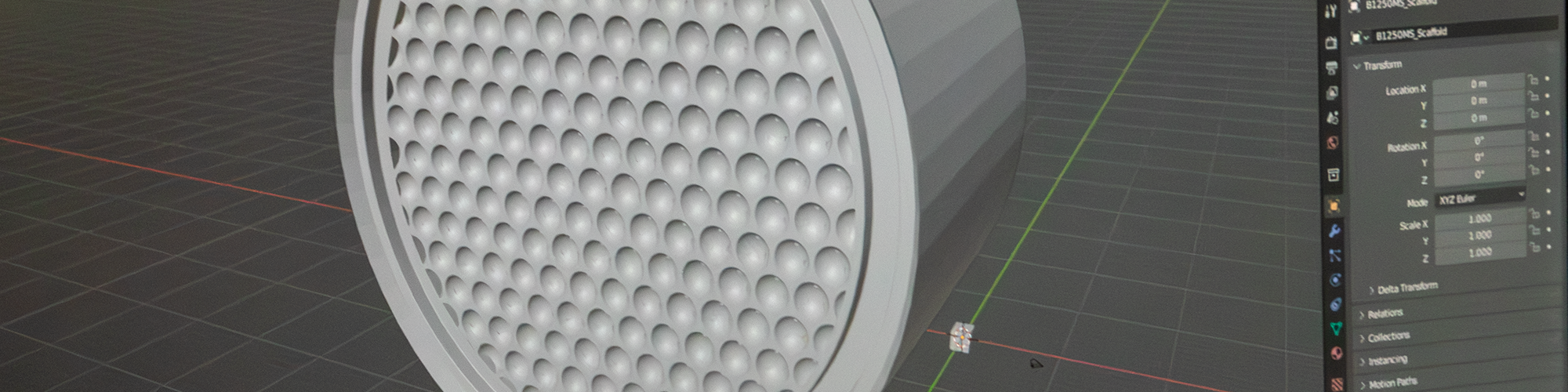
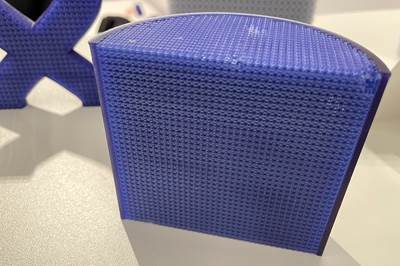
This is the bioreactor. This is a scaffold that goes inside of it. It has this complex geometry that could only be realized through 3D printing. And what this is about is getting enough surface area to grow adult bone marrow stem cells or peripheral blood stem cells for what is arguably the next frontier in medicine, using cell engineering to develop regenerative medicine treatments that show promise potentially for a range of afflictions, including Alzheimer's disease, diabetes, arthritis.
Stephanie Hendrixson
But the thing about studying cells like this and using cells for potential treatments is you need a lot of them. And typically the way that you cultivate enough cells today is by using lots and lots of these flat containers, these flasks. And so as the cells multiply, you have to keep dividing them, keep moving them in between containers. And so these 3D printed scaffolds are a possible solution, a way to get a lot more of that surface area into a much smaller size.
Peter Zelinski
To give you a sense of scale, it takes about a billion cells to get a quantity, a culture that is useful for one of these samples. But these cells, as they're growing, if they overlap, they'll begin to bond with one another. So you need enough surface to spread them all out. Complex, intricate geometry makes it possible to get all of that surface area into a small space to be able to do this efficiently. To learn more about this, we talked to Kreg Zimmern of the Southwest Research Institute. Kreg is senior research engineer involved with this project.
Kreg Zimmern
The Southwest Research Institute is an independent nonprofit contract research organization in San Antonio, Texas. We do everything engineering from, our quote is from deep sea to deep space. I work in the bioengineering of pharmaceuticals department and we do work in tissue regeneration and bioengineering to make medical devices, medical device development and things of that nature. So we see bioengineering and the cellular engineering, the stem cell stuff as kind of the next frontier in medicine. So it's biomanufacturing, right? So it's making biomolecules, proteins, enzymes, things of that nature with the cells as the product or as the manufacturing apparatus. So using your cells to actually manufacture something. Especially for stem cells, you need to have a large number of cells to accomplish those goals. And we are looking to address that problem. How do you culture enough cells and in a repeatable way and in a cost effective way. Our work is focused on generally adherent cells. So we started with stem cells, but we work can work with any type of cells that actually adhere to a surface. And the stem cells we use are adult stem cells and they usually are derived from either bone marrow samples or from what we call PBMC, peripheral blood mononuclear cells. Currently stem cells are cultured on flat T flasks. They look like just a flat piece of enclosed plastic that has a certain surface area. So say 75 square centimeters, 125 square centimeters, sometimes 225 square centimeters. If you want to spend a lot of money, you can buy the really expensive ones. And the cells are seeded onto the plastic they attach and then they grow. They split, undergo meiosis and mitosis. When you get to a certain number of cells, those cells start to touch each other on the flask, you have to take them off the flask so harvest them and split them into two more flasks in order to continue to grow them. So in order to continue to expand your population of cells, you need now two flasks and then four flasks and then eight and so on and so on. Until you get to the right number of cells that you need for your application.
Stephanie Hendrixson
So cultivating enough cells, using this process that Kreg described with all of these flat T flasks, it has some challenges. One, there's just a lot of hardware that's a lot of flasks that you need to purchase and keep track of. Two, it's a lot of labor. Somebody needs to go into the lab and keep dividing these cells as they continue to multiply. And every time you do that, every time you take your cell culture and move it from one container to another, there's a chance for contamination. There's a chance for something to go wrong. So these really intricate lattice structures that can provide that surface area that can be an alternative to those flat flasks mean that there are potentially just fewer pieces of hardware to deal with. There's less moving of the cells and it can be a more contained process.
Peter Zelinski
So talking to Kreg, it could easily take over a hundred of these flasks, well over a hundred, to grow a complete culture. This is an alternative. We have talked about the value of using 3D printing to increase surface area for heat exchangers, for example. This is an even more aggressive application because this very convoluted geometry that consists of all of these interlocking spheres, so small you can barely see them with your eye. That geometry creates enough surface area that a bioreactor like this potentially could replace scores of those flasks.
Kreg Zimmern
A bioreactor can be thought of from a high level as a chemical reactor. You know, a chemical reactor has a chamber where chemical reactions go on. The same thing for a bioreactor is a bioreactor has biological processes taking place inside of it. So our bioreactor is used for growing cells, which means the cell process of growing those cells or possibly secreting things from those cells is happening inside a closed system. The most important and kind of novel part of our bioreactor is the scaffold that we grow our cells on. And the scaffold can only be made through 3D printing. And we use a commercial level SLA vat polymerization 3D printer but using off the shelf resins.
Stephanie Hendrixson
We actually learned from Kreg that the material choice here isn't as significant as you might think. SwRI has actually developed some post-processing techniques to make these scaffolds pretty material agnostic, so there are potentially a lot of different resins that they could choose from for this application.
Peter Zelinski
The challenge with making these scaffolds, it's not the 3D printing, it's not the materials, it's not those physical aspects of the process. It is the digital part of the process. It is the large file size that comes from this complex geometry and the challenges of working with that geometry before 3D printing, even begins.
Kreg Zimmern
What makes this scaffold unique is it's got a very high surface area to volume ratio. So we have a very tightly packed bed of essentially spheres that we can get a lot more surface area and a much smaller volume. That means that the modeling was very kind of intensive. This isn't a hugely complicated scaffold. It's easy to understand. It's a repeatable structure, but it does involve a very large surface area, which means that the math that is being done to model it is extensive. So we use an enterprise level commercially available CAD engineering software. And the smaller bioreactors, so we have four sizes. We have one that's got 250 square centimeters of surface area. Those were not very difficult to model. Those, you know, were fairly straightforward. When you move to our largest bioreactor that's got 25,000 square centimeters of surface area, that becomes a much bigger problem. So there were a lot of hurdles to overcome with the modeling of these scaffolds, even though they are repeatable structures, like you would see in a lattice type work. The CAD files that we output are extremely large and hard to deal with computationally even when they're in their native CAD format. The export on our B-25000s, so the one with 25,000 square centimeters of surface area, was a 16 gigabyte STL. So that is an enormous STL that typically crashes lots of different types of printing software. We've been lucky. The vendor we use for our printing software that came with our printer has a very good software and they were able to handle it. But you can't really manipulate it. You got to get everything kind of set as it goes in, but a lot of other 3D printing software, so we've tried to, you know, to look at other printers. They can't even import the file. If they can import the file, they can't manipulate it and you can't generate your support structures. This file, the B25000 took me about a man-month to model, which is a very expensive model it was just computationally extremely heavy. A lot of that was waiting around to see if things would save. And so that really kind of bogs you down and you're never really sure, you can't be predictable in the way that you're working. Your workflow is extremely disrupted when you have files like this. I can't send them to other people to say, do test prints for me, or if I want to outsources to a service bureau to get a print and a new material that a lot of times it's not possible with the large files, they just can't handle them. It makes everything you do very fragile on that end, which is a big problem when you are talking about making these in production.
Peter Zelinski
And production is where this is headed. The bioreactor works. Kreg and his team have demonstrated they can design and scale these scaffolds. They can produce them postprocess them, they can grow cells on them. What comes next is scaling this up, scaling up the scaffolds, for example, to like a size that might be suggested by these sections or bigger. A size that can do industrial scale cell growth. That's what's needed here. And from a hardware standpoint, this is very doable. There are proven 3D printing systems that have a work volume large enough to accommodate a part like this. The challenge instead is the math. It is the geometry. Increasing the size of the scaffold multiplies that already really big geometric file size and multiplies it to a size that it becomes impractical, impossible even to work with in the CAD tools, even sophisticated CAD tools that Southwest Research Institute has available. So that's where the team is right now. That's the impediment that they face. And it's more than just a design and modeling challenge because it affects the 3D printing too.
Kreg Zimmern
So, you know, you've got to have everything dialed in and then maybe you can do production. The other part of this is right now we have a commercial grade SLA vat polymerization machine, and the large scaffold take 250 hours to print, and that's a continuous 250 hours. So again, it's a case of I started it and I hope there's nothing that goes wrong in that 250 hours until it comes out. The process is scalable and is only limited now by our hardware and our software. We don't think it is no longer being hampered by just the biological process, which is our general hurdle. But it's the hardware, the 3D printing hardware, so the printers and it's the software, the modeling software and the slicing software that is kind of our big hurdle. And being able to scale this to generally almost whatever scale we think we want to use. So picture this in a giant, you know, 250 gallon reactor to grow lots of cells or to make things like insulin, to make things like vaccines, to make things like that that are bio produced that we could produce at scale and quickly.
Stephanie Hendrixson
So the scaffolds that we have here with us in the studio can be produced on the equipment that SWRI currently has. They've been tested in bioreactors. They're working as expected. Now the next phase of the project is that scaling up, getting to a larger size for the scaffolds, for the bioreactors to get to larger quantities of cells. We've got more to say about scaling up in a minute. But before we go any further, I think we should recap.
Peter Zelinski
Okay, recap. So bioreactor, and the principle 3D printed component of it is this scaffold that has the intricate geometry, geometry that realizes a surface area making it possible to grow millions of cells, maybe billions of cells in order to get the right culture size.
Stephanie Hendrixson
These scaffolds are 3D printed through Stereolithography. They are postprocessed to make them suitable for cell cultures. SWRI has demonstrated that this idea works, they've been able to manufacture these scaffolds to test them in bioreactors, and now they're looking towards greater and greater scale.
Peter Zelinski
Getting to greater scale. So the challenge of that, it's a math challenge. It is the difficulty of defining this intricate geometric form. And we've got a little more to say about that because we met a company that is working on exactly this challenge, modeling the kinds of forms that additive manufacturing can realize.
Stephanie Hendrixson
That's right. We spoke with Elissa Ross. She's a mathematician and the CEO of Metafold, which is a software company providing solutions for implicit modeling. And Elissa explained that a lot of CAD systems work by directly modeling the surfaces of a part. For a lot of parts that works just fine. But when you're faced with something like this where the goal is literally to put as much surface as possible into a volume, it creates some challenges.
Elissa Ross
CAD systems typically work by modeling the surface of a shape, and they do this either using what's called a boundary representation, often called a B rep, or with a collection of very small triangles, which is a triangle mesh. And people often interact with triangle meshes in the form of STL files. And this is actually fine for most manufacturing methods. If we think about things we try to produce with conventional manufacturing, you don't need a lot of surfaces to represent a lot of those shapes. Not the case for 3D printing, because in 3D printing we have this new opportunity to create very high surface area structures with lots of fine details. And if you're doing that and you think about trying to represent every piece of surface in these high complexity structures, you're talking about a lot, a lot of triangles. And you may find actually with some of these shapes, you can have a STL file that can be many, many gigabytes.
Peter Zelinski
So with an STL file, you're taking the intricate geometry and you're defining it using triangles, all the triangles it takes. Metafold uses a different approach in their system. They use implicit modeling to define the form. The form is implied using equations that are capable of capturing the geometry using a lot less data. Here's Elissa again.
Elissa Ross
Implicit modeling is a different approach. It relies on math, literally equations to describe the surface of an object. So it's a very elegant and succinct way to describe and communicate shape. It doesn't involve these massive files that contain all these details of surfaces. So more specifically, implicit modeling typically relies on what's called a signed distance field. And this is effectively you can think about it as a grid. And in every point on the grid, there's going to be a piece of data that says how far away is that point from a shape? What's nice about this is that it's very precise, very accurate. It's also extremely robust. And we get all kinds of incredible geometric operations along with it kind of for free. So the key idea of implicit geometry, representation is that our shapes are more like recipes than surfaces.
Stephanie Hendrixson
So moving from explicit modeling to an implicit modeling strategy has some advantages. The file sizes get smaller, design changes are faster and easier to execute, and the user experience is probably going to be a lot more streamlined.
Elissa Ross
Honestly, if we are succeeding, then a user won't really know or care how the geometry is handled under the hood. Whether it's explicit or implicit, they just know they're getting shapes on screen really fast and really easily.
Peter Zelinski
So we've seen that as in the case of the bioreactor scaffold, additive manufacturing is realizing geometries that challenge CAD software, but CAD is adapting to keep up.
Elissa Ross
There are so many exciting applications of additive manufacturing where I think implicit modeling is more than valuable, it's actually critical to getting these projects executed. Let's say a heat exchanger where you're trying to maximize the surface area between a hot thing and a cool thing. For example, anything to do with filtration or carbon capture. You're trying to put as much, let's say, air containing carbon. You're trying to put as much of that in contact with a surface that has absorbent characteristics for absorbing carbon from the air.
Related Content
6 Trends in Additive Manufacturing Technology at IMTS 2024
3D printers are getting bigger, faster and smarter. But don’t overlook the other equipment that the AM workflow requires, nor the value of finding the right supplier.
Read MoreWhat Does Additive Manufacturing Readiness Look Like?
The promise of distributed manufacturing is alluring, but to get there AM first needs to master scale production. GKN Additive’s Michigan facility illustrates what the journey might look like.
Read More3D Printing with Plastic Pellets – What You Need to Know
A few 3D printers today are capable of working directly with resin pellets for feedstock. That brings extreme flexibility in material options, but also requires greater knowledge of how to best process any given resin. Here’s how FGF machine maker JuggerBot 3D addresses both the printing technology and the process know-how.
Read MorePossibilities From Electroplating 3D Printed Plastic Parts
Adding layers of nickel or copper to 3D printed polymer can impart desired properties such as electrical conductivity, EMI shielding, abrasion resistance and improved strength — approaching and even exceeding 3D printed metal, according to RePliForm.
Read MoreRead Next
Implicit Modeling for Additive Manufacturing
Some software tools now use this modeling strategy as opposed to explicit methods of representing geometry. Here’s how it works, and why it matters for additive manufacturing.
Read More3D Printed Heat Exchanger Uses Gyroids for Better Cooling | The Cool Parts Show #43
Replacement heat exchanger for a helicopter is half the size and delivers 4× the cooling, thanks to a geometry that could only be made via additive manufacturing.
Read MoreSpherene Creates Metamaterial with Geometry Derived from Spheres
An algorithm developed by Spherene Inc. generates Adaptive Density Minimal Surfaces (ADMS) as a self-supporting infill strategy that can be used to reduce mass and manage material properties in 3D printed parts.
Read More