Researchers Developing Scalable Manufacturing for Novel Vaccine Delivery
Hybrid-MNA technology may significantly help reduce vaccine shortages.
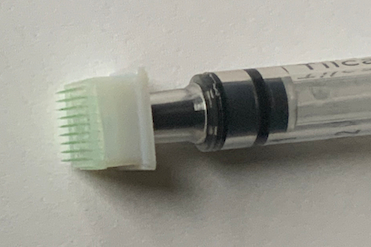
The novel Hybrid-Microneedle-Arrays require as little as 1/100th of the dose of traditional vaccines.
Collaborators led by Carnegie Mellon University are developing a novel approach to COVID-19 inoculation that addresses both immunological effectiveness and manufacturing efficiency with a low-dose, inexpensive, hybrid microneedle array (Hybrid-MNA) technology.
The Hybrid-MNA is a new, intradermal delivery device that builds on more than a decade of work on microneedle array technology led by Burak Ozdoganlar, professor of mechanical engineering at Carnegie Mellon University and the project’s principal investigator.
The novel vaccine delivery method enables a very small amount — potentially 1/100th of the dose of a traditional vaccine — to elicit strong and long-lasting immunity against SARS-CoV-2 infections. This can significantly help to reduce vaccine shortages.
A second focus of the project is optimizing and automating the production process for the Hybrid-MNAs. This will include using 3D printing during the fabrication process and robotic automation during production. Both will ensure efficient and inexpensive manufacturing of the devices, the collaborators say.
Because the Hybrid-MNAs do not require the same level of cold-chain storage as other vaccines, they will be more easily transported and stored.
This two-fold approach to tackling the COVID-19 pandemic earned the multidisciplinary team a $643,359 grant from the Commonwealth of Pennsylvania. In addition to Carnegie Mellon, collaborators include the University of Pittsburgh Center for Vaccine Research and industrial partners Boston Micro Fabrication (BMF), Premier Automation and Tiba Biotech.
“Our team brings a proven track record of multidisciplinary expertise and experience in immunology, vaccine design, development and delivery, 3D printing and industrial robotic-automation,” says Ozdoganlar, who is also the associate director of Carnegie Mellon’s Engineering Research Accelerator.
BMF believes the project will improve vaccine delivery. “Our micro-precision 3D printing technology allows for the rapid and precise fabrication of micro needle arrays, resulting in time and cost savings that far exceed traditional manufacturing methods,” says John Kawola, CEO of Boston Micro Fabrication.
Related Content
-
Q&A With Align EVP: Why the Invisalign Manufacturer Acquired Cubicure, and the Future of Personalized Orthodontics
Align Technology produces nearly 1 million unique aligner parts per day. Its acquisition of technology supplier Cubicure in January supports demand for 3D printed tooling and direct printed orthodontic devices at mass scale.
-
What Does Additive Manufacturing Readiness Look Like?
The promise of distributed manufacturing is alluring, but to get there AM first needs to master scale production. GKN Additive’s Michigan facility illustrates what the journey might look like.
-
Airless Basketball Shows Promise of 3D Printed Lattices: The Cool Parts Show Bonus
Successfully matching the performance of a standard basketball demonstrates the control possible over the mechanical properties of digital materials.










