Founded in 2004, medical molder Medbio, LLC (Grand Rapids, Michigan) operates four facilities in the U.S. equipped with a total of 119 thermoplastic and two liquid silicone rubber (LSR) injection presses in ISO Class 7 or 8 certified cleanrooms. Together with its parent, plastics caps and plugs manufacturer Protective Industries Inc. (Buffalo, New York), the combined company operates 17 molding facilities globally.
To support active programs, Medbio produces or commissions 100 tight-tolerance injection molds annually to produce medical devices and diagnostic equipment. It’s no surprise that the company has been using polymer 3D printing — much of it conducted in house — for the last 5 years to produce prototype parts for new programs and after engineering changes, as well as for fixtures to support its extensive molding operations.
Interestingly, the company sets aside CAPEX funding every year for technology development, which enables Medbio engineers to purchase and try new equipment that might prove useful. That’s how its Grand Rapids team started working with polymer AM technology. It presently owns an FDM/FFF printer from Stratasys on which it prints polycarbonate/acrylonitrile butadiene styrene (PC/ABS) and PLA, and an SLA printer from Formlabs on which it prints epoxy.
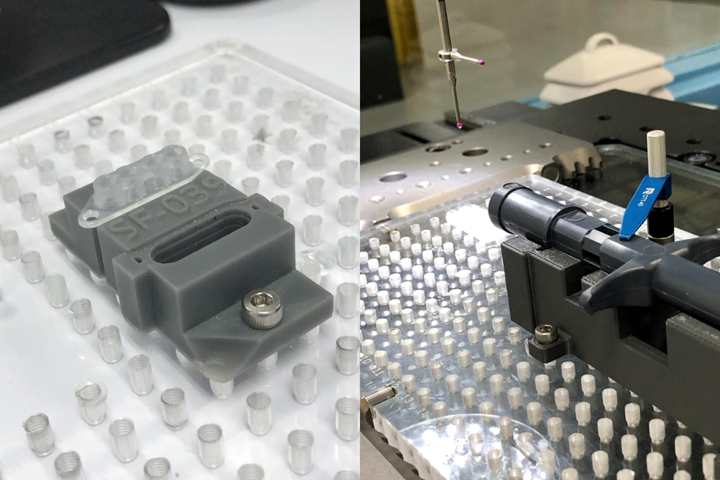
The prototype EOAT shown at the top was printed via FDM/FFF and is designed to grab two different parts from an inline escapement off of a vibratory feeder bowl and move them to the rotary dial in an automated laser welding cell. A second prototype EOAT shown below features a diagnostic device (left side) that has been picked up. This tool was designed to attach to a selective compliance assembly robot arm (SCARA). Photo Credit: Medbio LLC
“We primarily use our printers to test out jigs and fixtures to see if our concepts will work, as this gives us something we can hold, feel and turn around in space, plus we can quickly and inexpensively try multiple different versions of a design and see which works best,” Ethan Bruyn, Medbio manufacturing technology leader, explains. “Given the molding tolerances we have to maintain — often as tight as 0.002 inch [0.05 millimeter] — we have a large metrology department, so we do a lot of work developing holding fixtures, CMM fixtures, along with jigs for our assembly team. Once we’re sure a design will work, for the most demanding applications where anything that touches a molded part is required to have FDA approval, we either reproduce the design in anodized aluminum or stainless steel or machine it from a block of PEEK [polyetheretherketone]. For less sensitive programs that don’t require an FDA-approved material for part contact, we may keep the fixture in polymer additive.”
He notes that most of the company’s EOAT are produced in metals because of the amount of wear they experience on high-volume molding programs.
“While many companies are pushing 3D printed EOAT for everything, we like to assess each application and base the design and construction off the part, cycle time and degree of complexity required,” Bruyn adds. “We have fully 3D printed EOAT that have work out well, but we’ve had others where the frequency at which wear components needed to be replaced limited our ability to use the technology — especially when we needed to thread into plates. On the other hand, we’ve also had a situation where a conventional metal component for an EOAT was lost and, due to the long lead time for machining a replacement, we turned something around on our Stratasys in a day to get out of a bind versus waiting 2 weeks for a replacement to be machined. Overall, I think 3D printing has evolved a lot in recent years and I’m very curious to see what happens in the next 5-10 years.”
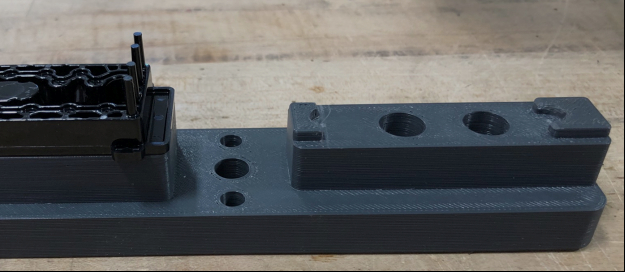
Above are two CMM inspection nests. The one on the left was printed on an SLA printer and is for a diagnostic device, while the one on the right was printed on an FDM/FFF printer and is for a hybrid metal/plastic surgical instrument. Photo Credit: Medbio LLC
More From This Author
Peggy Malnati is a Detroit-based contributing writer for CompositesWorld magazine, for which she primarily reports on automotive and ground transportation. SUBSCRIBE HERE
Related Content
Robot Vs. Gantry for Large-Format Additive Manufacturing (Includes Video)
Additive Engineering Solutions, specialist at 3D printing very large parts and tools on gantry machines, now also uses a robot for large-format AM. Here is how the robot compares.
Read MoreMantle: 3D Printed Molds Address Plastics Industry Lead Time and Skills Shortage
Company now shipping production systems. Steel mold tooling from its TrueShape process can be printed, shaped and sintered in days, and with fewer steps, compared to weeks of lead time for molds made conventionally.
Read More8 Ways the Plastics Industry Is Using 3D Printing
Plastics processors are finding applications for 3D printing around the plant and across the supply chain. Here are 8 examples.
Read MoreThe Connector Conundrum: 3D Printed Mold Tooling’s Role in Innovation
ReelView Fishing faced an electronics obstacle in the development of its new technology for underwater video. Additive manufacturing for moldmaking allowed for the speed necessary to iterate to a solution. How inventors and invention will benefit from new ways of obtaining production-ready tooling.
Read MoreRead Next
Understanding PEKK and PEEK for 3D Printing: The Cool Parts Show Bonus
Both materials offer properties desirable for medical implants, among other applications. In this bonus episode, hear more from Oxford Performance Materials and Curiteva about how these companies are applying PEKK and PEEK, respectively.
Read MoreAutomated 3D Printing at Evco: Composites, Cobots, Email and More
Injection molder Evco has long seen the importance of industrial automation for plastics processing. Its latest automation feat? A cobot-tended cell of 3D printers for manufacturing fixtures and customer products unattended.
Read MoreCustom Molder Applies Polymer, Metal 3D Printing for Productivity Gains
Custom injection molder Westec Plastics Corp. uses 3D printed end-of-arm tooling for automation as well as inserts printed in metal.
Read More

.jpg;width=70;height=70;mode=crop)