3D Printing and Coronavirus Check-In - Week of 3/30/2020
In this week's discussion, editors Peter Zelinski and Stephanie Hendrixson discuss face shield visors and masks; ventilator components; and new movement from the FDA on 3D printed devices.
Since last week’s check-in, there have been several significant updates on 3D printing’s role in the fight against coronavirus. In this video, we discuss immediate needs and applications for the healthcare industry as well as long-term implications for additive manufacturing as a result of COVID-19.
Topics addressed in this discussion:
- face shields with 3D printed visors
- viability of 3D printed reusable N-95 masks
- ventilator splitters and masks
- FDA guidelines and emergency use authorization for 3D printed items
- efforts and resources to match hospital needs with 3D printing capacity
If you are a hospital using 3D printed PPE and can spare a few minutes to talk about your experience, we’d like to hear from you: Email us.
Transcript
Peter Zelinski
I’m Pete Zelinski with Additive Manufacturing dot Media.
Stephanie Hendrixson
I’m Stephanie Hendrixson, also with Additive Manufacturing dot Media.
Peter Zelinski
So much is happening in the overlap of 3D printing and the coronavirus crisis. We decided a quick, untidy, rough, roughly once a week video conversation is maybe the best way to process it all in real time. Stephanie, we've been getting new viewers new audience since this crisis started. Can you give a quick intro who we are what we do?
Stephanie Hendrixson
Yeah, sure. So Additive Manufacturing is a B2B publication that focuses on industrial applications for 3D printing technology. We have a website, a newsletter, a print magazine, we have YouTube videos, we have a series called The Cool Parts Show. And we are all about industrial uses of 3D printing technology. So that could be anything from prototypes and assembly tools through aerospace components, medical implants, even consumer goods like 3D printed shoe insoles. So covering a really broad range of things, but it all ties back to real, functional uses for 3D printing.
Peter Zelinski
Some of the new audience that's finding us is medical providers looking for 3D printing, maybe for personal protective equipment, which I think we're about to talk about. But maybe also just to find some component to keep some vital system up and running during this time. We've got an article at Additive Manufacturing dot Media that speaks to this group. It's near the top of the home page. You can find it there. But to dive into what's happening in 3D printing in the midst of the coronavirus crisis. Stephanie, what's the first topic we should talk about?
Stephanie Hendrixson
Yeah, so you mentioned PPE, and I think that's kind of where we'll dive in. So we talked a little bit last week about face shields, and there's been some some movement there. So last week, you mentioned Stratasys, and I think even the day that we filmed our last conversation, they shipped their first batch of face shields to hospitals. We've seen similar efforts to organize users from companies like HP and 3D Systems. We're seeing a lot of really big manufacturers get involved in this. So Ford, for example, is now 3D printing visors and assembling face shields.
Also on the PPE front, we talked about 3D printed masks as an N-95 alternative. And last week, there wasn't really a good design out there, it didn't seem, but from what I've what I understand that seems like it's changing. What are you seeing Pete?
Peter Zelinski
Right, so face shields and masks, two different components of PPE that is pretty fundamental and vital for healthcare workers dealing with COVID-19 suffers. So the face shield is simple product. It's the visor that holds the clear face shield in place and then the shield itself the visor is the 3D printed part of it shield can be cut from like mylar or PET. The reason why this could be produced quickly by lots of different providers and makers through 3D printing is because no FDA approval is needed for a device like this.
But what we are seeing is different hospitals in different regions, different hospital systems have different preferences about the facial design that they're willing to accept, related to how much of a crisis they're in with this equipment. For some of them like for example, protecting the the upper part of the head is important and other designs sort of let go of that requirement.
We are seeing and hearing, to your point, Stephanie, is kind of some secondhand reports of how 3D printed personal protective equipment is starting to arrive at hospitals is starting to be used there. We hope next week when we do this video to be able to confirm that and to have heard from some hospitals that are starting to put that into use.
Connect with us, reach out to us if you are in this effort if you're directly supplying hospitals now, and have users who can talk to us quickly and the reason we want that is because we want to showcase working models of the looping all the way close the hospitals needs being met for other hospital systems that are ramping up and looking for the way to provide for their own needs.
As the the suffering from this virus moves to different parts of the country, we're starting to hear reports of material shortages: 3D printing filament, or material for the shields, which—the shields themselves aren't 3D printed, I should maybe maybe specify that they're cut. The 3D printed part of it is the visor. But in both of those components, we're starting to hear of material shortages. And we might see that hospitals are better served in the areas where just the makers in that area and the companies got involved more quickly and bought up the material more quickly.
Which makes it worth saying: If you are not making an approved PPE design, if you're not making something that is already been ordered by a hospital and is destined to be used by a hospital, think twice, maybe stop, because it could be that you're using material on an effort that might not completely close the loop on what's needed.
And you also Stephanie mentioned masks; that situation’s harder. So the N-95 mask, it's the typical mask, it is a filter that's just in the shape of a mask and the companies that produce those kind of masks, the big companies like 3M, they're ramping up production, that's at least going to partially solve the need. But the alternative is yet 3D printed reusable mask with a replaceable filter. So this needs FDA approval because it has to pass a pass a fit test to make sure it protects the wearer and we are close to FDA approval on a 3D printed mask design. It has passed fit tests initially, there's one more test it's being evaluated for. It uses a HEPA filter that you can get in a hardware store as the replaceable element.
If there's another widely available design for a 3D printed mask usable for hospital PPE, we're not aware of it. Contact us if there is but I think we're very close to reporting on a widely shareable 3D printed mass design.
Stephanie Hendrixson
Okay, so a 3D printed reusable mask with that kind of readily available consumable, how far along are they realistically with this? Right?
Peter Zelinski
So they've done fit tests that have succeeded on like four very different face geometries. And that my understanding is that has passed the requirements that needs to pass. There's one more test and it relates to it's it's a splash test, It relates to that possibility and making sure that level of protection is there to the FDA. What we're hearing is being very responsive and very accommodating to getting to allowing to get new ideas into use quickly, as long as it can be assured that the user who believes they're going to be protected is protected. I think we're probably going to get it there's a lot more to say about the FDA. I think we're gonna get there.
The other big manufactured part of this is ventilators. Right? Are you hearing anything about ventilators, Stephanie?
Stephanie Hendrixson
A couple of things. So taking this outside of the United States for just a second, Isinnova, which is the company that we briefly touched on last week that had 3D printed the the ventilator valves, is now working on a design to convert a snorkeling mask into a ventilator mask. So one of the one of the potential treatments if you have the coronavirus if you do need a ventilator you might need to be intubated where they put tube through your mouth or your nose to help you breathe, or, or there's a mask option as well. And so what they're working on is a way to convert these, these snorkeling masks into a ventilator mask that would go over a person's face. And it's it's just a 3D printed component that they attach that allows the, the airflow to come in and out and they're calling it the Charlotte valve. Again, not something that we're seeing in the U.S. yet, but it kind of maybe is a preview of the kinds of solutions that we may be looking at in the future.
Regarding FDA certification, so you kind of touched on the fact that we're seeing some some regulations loosening up a little bit. Just after we spoke last week, you wrote about the VESper ventilator expansion device, which is basically a splitter allowing you to put up to four patients on the same ventilator, which seems like a really promising solution, but from what I understand there's a little bit more to it than just kind of plugging and playing with it. Do you want to talk about some of the issues in using splitters like that?
Peter Zelinski
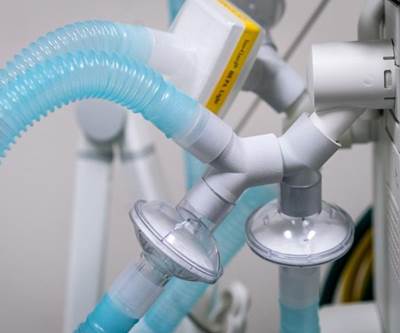
Yeah, so the ventilator so this is the hardest manufacturing challenge of all and maybe the item that is most urgently needed and all of this and 3D printing can't solve that problem. The ventilator shortage, this is a manufacturing challenge, bigger than additive manufacturing. You know, we're seeing the news about ventilators right in the arithmetic is pretty harrowing. In New York, the governor of New York, I think a couple times has said the the shortage there the need there is 30,000 ventilators right away. I just saw in the news, Ford and GE are in a partnership and their aim is to produce 50,000 ventilators by early July, which is not right away. Dyson the vacuum cleaner maker is apparently designed a ventilator and is ramping up production of it. of them. GM is involved in a in a partnership related to ventilators. MIT released a model for a very simple ventilator a very simple model design that maybe could be produced more quickly. So this story is still unfolding and we'll see if the ventilator need is met. We earnestly hope that it is. But meanwhile, additive manufacturing is playing a pretty critical role in the short term. FDA, as you mentioned, gave emergency approval for a ventilator splitter allowing up to four patients at once to use one shared ventilator. The design came from Prisma Health. It is produced on HP’s Multi Jet Fusion 3D printing platform. There are some signs that some other hospital systems are using some other splitter designs that may or may not have the formal FDA recognition. This isn't clear.
But what the device does is very straightforward. It's basically plumbing. It branches off the tubing from the ventilator so that it can go to multiple patients serve multiple patients, and then the doctors to make this work. Doctors find patients that are roughly equivalent in terms of their need, in terms of their lung compliance, in terms of the ventilator settings that they need. And they pair them together and they they connect them to the same ventilator as a way to multiply ventilator capacity. And then it becomes sort of a fluid mechanics problem. =You don't set air flow rate as the target for the ventilator to hold as you might in a normal situation, because if you do that, then if there happens to be a blockage in one tube, then the other patient gets all of that flow rate. So instead, you set a target pressure for the ventilator to maintain. You monitor the patients independently using monitoring tools independent of the ventilator because the ventilator doesn't expect to be used this way. And then you you watch each patient for consistent airflow.
I heard one physician talking about this — he noticed that in trying to use this there's drift over time and as sort of one patient ends up getting more of the airflow and so his solution was he puts resistance into one of the tubes, puts things inside the tubes to try to balance out the flow. Like it's that sort of like, , you know, sort of work it out on the fly-type care that this solution requires. But it is a solution. It's a solution for now.
Another part of the solution is you keep some ventilators on hand beside the shared ventilators, for patients for whom this isn't just, this just isn't working emergency single use ventilators to be brought in for patients who can't do the sharing. I heard a doctor talk about his sense of what this is what this option is. He says like this is the this is the best option we have while we wait for the cavalry to come. That was his phrase, “wait for the cavalry to come in.” In this case the cavalry is manufacturing the ability to produce a lot more ventilators.
And the FDA is doing things that maybe we wouldn't have expected them to do, wouldn't expected to see them see as their role to play. You've been kind of looking at this, Stephanie, and you've had a chance to kind of be close to this and think about this. What role is the FDA playing? And what are you seeing?
Stephanie Hendrixson
Yeah, so, I've had a lot of people ask me recently, and I'm sure you have too, you know, if if I've got open 3D printing capacity, what can I make? How can I help? Who do I go to, for for direction and for designs and and to work through the FDA process?
So yesterday, I sat in on a webinar with American Makes, and they are trying to be kind of a central repository of information for the 3D printing community right now. And so they've partnered with the VA, with NIH, and with the FDA to put out this collection of resources online. So they have a new COVID-19 page on their site that you can visit and it has these different portals. There's one for healthcare. So like hospitals and clinics, and that needs supplies. There's one for manufacturers. So if you have open capacity, you can input your capabilities there. And then there's one for designers.
And there's a couple of purposes for the the resources that they're putting out there first is they're trying really hard to match healthcare needs with suppliers who can who can handle that. So if you are a hospital, procurement officer say, and you go in and enter the things that you need that could be potentially 3D printed through this form. What America Makes is doing on the back end is then trying to match your need with one of the manufacturers in their database. And so it's not it's not a marketplace, they're not going to broker any of the any business that comes out of this. What they're doing right now is just presenting the healthcare customer with some potential manufacturers and kind of leaving it up to them to to negotiate and reach out and kind of work that deal offline, but it's becoming a good repository for, here are the people with the real needs. And then here are some manufacturers who can who can help with those needs. So being that matchmaker.
The other thing that I think is really interesting that they're doing is that design portal. So they're letting anybody kind of go through there and and upload designs related to healthcare items, related to PPE. All those designs are going to end up on the NIH’s 3D print, open source directory. So there are all these designs out there. But once you submit something through that portal, what happens is kind of interesting. So they've set up this whole workflow with the involvement of their partners.
Once the design is uploaded, the VA kind of takes the first look at it and decides, okay, is this something that is that is needed, that's going to be useful? And is it viable? And if the answer is yes, the next question that they asked is okay, is this one of those items that's going to need FDA oversight. So is it a ventilator component, something like that? An N-95 mask? Or is it something more like a face shield where we can proceed without that sort of certification process? If it doesn't need the FDA, then at that point, it can go into clinical trial in a VA hospital. But if it does need the FDA approval, at that point, the FDA would get involved directly to help expedite the qualification process, or grant that emergency use authorization if that's the case. And so I think this is a really good example. People have been asking for looking for guidance on this. And here's what's becoming a pretty direct path to design the part, and work through the process to get it certified if it's something that can be used. It's a pretty direct and expedited way to get that needed part out there.
And then all those designs are going to be living on that repository. They're all going to be labeled so you'll know what stage they're at if it is something that's that's in that approval process, or if it's just kind of a prototype, and it's just an idea at that point, but I think we're seeing some good responsiveness from the FDA and recognition that 3D printing could be a potential solution. There's also been some some new documents and things on their website lately. So for instance, just last week, we spotted the frequently asked questions that they put out there for 3D printing professionals. Do you want to talk a little bit about that?
Peter Zelinski
Yeah, that's the guidelines that were in the last couple of days FDA put out guidelines on 3D printing medical devices and accessories, particularly in the context of the COVID-19 crisis. And I just, I think that that is a historic document. The crisis-level tone of this comes through in that document, the scale of the emergency.
It's seen, for example, in some of just the related resources that that document links to. The frequently asked questions sort of speak to what healthcare providers can and cannot expect from from 3D printing. Are 3D printed masks automatically safe and automatically providing protection for the wearer? What is connected to that is the FDA guidelines for managing in the situation of a shortage of N-95 masks and sort of an escalating scale of what it's okay to accept depending on how bad the shortfall is, all the way to the point of an FDA documents saying use a bandana, wrap a bandana around your your mouth and nose to just as protection if that's the best that there is. Just the acceptance that some some extreme emergency measures are might be needed here and and what is acceptable.
Another reason that those guidelines are important is because they speak to what 3D printing can and can't do for hospital leaders who hope that it will be the answer. And this is important because buried in that document, in the in the assumptions underlying those guidelines, are clues to what we'll need to talk about soon. What manufacturing should be able to do, what it can do if we have the will to go there. Right now we need to address the immediate need. But there's all kinds of lessons we're learning here about how to create a manufacturing system that can respond quickly. And if we learn those lessons well and implement those lessons, well, we'll be way better prepared for the next crisis.
Stephanie Hendrixson
Yeah, I agree. I think there's a lot of long-term implications of this situation and the way that we're handling it, and it's going to it's going to change manufacturing. But in the short term, I think we still have some really big questions to answer. For us specifically, you know, we keep seeing these reports of 3D printed face shields and and other equipment that's going somewhere and we haven't really been able to close the loop and and get to talk to a hospital or some other healthcare provider that's using 3D printed equipment. I think that's something that we'd really like to hear from somebody who can tell us about that so we can illustrate, you know what it looks like when when the system is working successfully.
Peter Zelinski
That's right, and our intent is not to get in the hospital's way. They're very busy right now. And it can be a short conversation. But we want to showcase models where the loop has been all the way closed and the hospital’s need has been met. Because different hospital systems are proceeding through this crisis at different times in different parts of the country. And a model that works well in one place could be quickly replicated someplace else.
We have some resources available on our website, Additive Manufacturing dot Media for medical providers. We have an article on the site describing how to engage with 3D printing service providers, how to find them, what questions to ask what to expect, you can find at the top of our site.
For manufacturers, we have a map to 3D printing service providers for help in dealing with short term production needs and interruptions during this time, which is another aspect of this situation that continues to play out.
Stephanie Hendrixson
Right, so we are planning to be back next week with another update like this. In the meantime, you can follow us both on on social media and check out additive manufacturing dot media for more 3D printing information, both related to the pandemic and otherwise. So we'll see you soon.
Related Content
Why AM Leads to Internal Production for Collins Aerospace (Includes Video)
A new Charlotte-area center will provide additive manufacturing expertise and production capacity for Collins business units based across the country, allowing the company to guard proprietary design and process details that are often part of AM.
Read MoreHow Machining Makes AM Successful for Innovative 3D Manufacturing
Connections between metal 3D printing and CNC machining serve the Indiana manufacturer in many ways. One connection is customer conversations that resemble a machining job shop. Here is a look at a small company that has advanced quickly to become a thriving additive manufacturing part producer.
Read MoreHow Norsk Titanium Is Scaling Up AM Production — and Employment — in New York State
New opportunities for part production via the company’s forging-like additive process are coming from the aerospace industry as well as a different sector, the semiconductor industry.
Read MoreWhat Is Neighborhood 91?
With its first building completely occupied, the N91 campus is on its way to becoming an end-to-end ecosystem for production additive manufacturing. Updates from the Pittsburgh initiative.
Read MoreRead Next
Guide for Health Care Providers: How to Find (and Talk to) 3D Printing Professionals
If you are a medical professional in the U.S., you've likely heard that 3D printing can be an answer to equipment shortages. Here's how to find local 3D printing specialists and how to talk to them once you have.
Read More3D Printed Device Allows Multiple Patients on One Ventilator
Emergency authorization from FDA allows for use of device permitting four patients to use a shared ventilator. HP involved in mass production via additive manufacturing.
Read MoreNeed Parts? These Additive Manufacturers Are Ready to Help
Disrupted supply chains are just one more effect of the coronavirus pandemic. These 3D printing service providers are ready to help fill production gaps with parts, tooling and prototypes.
Read More
.jpg;width=70;height=70;mode=crop)