3D-Printed Molds Accelerate Substitute-Ventilator Validation
Fortify’s 3D printing and injection molding expertise saved Ventilator Project weeks and thousands of dollars as the nonprofit hurried to supply clinicians and hospitals at the start of the COVID-19 pandemic.
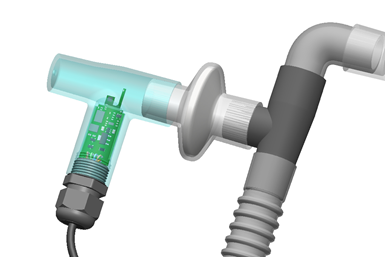
Ventilator Project, a Rhode Island-based nonprofit sourcing alternative ventilator solutions to meet clinician and hospital demand during the coronavirus pandemic, obtained thousands of donated sleep apnea machines (such as CPAP and BiPAP devices) to serve as supplementary equipment for hospitals. Once the organization sent these machines to doctors to establish a protocol for use, however, Ventilator Project received feedback that the machines needed a disconnect alarm. This critical feature would alert clinicians if airflow to the patient was interrupted at any point and establish these home-use machines as suitable ventilator substitutes for hospitals.
Ventilator Project designed a T-splitter component to house the alarm, but needed to create a prototype of the final device to test in an oxygen-rich environment to receive FDA approval. This became a materials problem: the nonprofit needed to conduct efficacy and safety validation testing on the part and end-use manufacturing material — in this case, polypropylene — before it could scale up production.
Injection Molding and 3D Printing Challenges
While 3D printing could have delivered a prototype to confirm the fit, form and basic function of the T-splitter, achieving FDA approval for a medical device requires the manufacturer to validate a test component made from the end-use material using the end-use manufacturing process. Although suppliers now offer polyproplyene as a 3D-printing material, Ventilator Project knew it would ultimately make the splitters via injection molding. Validating the design would therefore require an injection molded, polypropylene T-splitter, and obtaining the tooling could cause an especially sizable impact to the project’s cost and timeline. With the ongoing nature of the coronavirus pandemic making time a precious resource and the project’s minimal budget limiting its options, Ventilator Project could not proceed with traditional manufacturing methods.
To solve this issue, Ventilator Project partnered with Fortify, a digital manufacturing company based in Boston. Ventilator Project came to Fortify with two T-splitter designs that it wanted to test.
Lowering Costs with a 3D-Printed Injection Mold

Rather than design and build two full conventional molds, Fortify’s applications engineering team designed and 3D printed one mold tool with two sets of hand-loaded core inserts that were capable of producing two unique geometries from that mold. The molds were made on the company’s in-house Flux One 3D printer platform from its Digital Tooling resin through a combination of magnetics and digital light processing (DLP) 3D printing. The Flux One’s Digital Composite Manufacturing (DCM) technologies – Continuous Kinetic Mixing (CKM) for even mixing of resins and additives, and Fluxprint for magnetically aligning reinforcing fibers across each 3D-printed layer – resulted in greater control over the final geometries of the Digital Tooling-comprised inserts while increasing their durability.
The team’s ability to print the inserts simultaneously alongside the mold added no extra time to the build and only added an additional $30 to the cost.

Especially important in saving time and cost was the ability to 3D print threaded inserts. Threads are typically challenging to prototype due to long lead times — even an off-the-shelf insert can take one or two weeks to source, and several quotes for the custom thread Ventilator Project needed clocked in at six weeks. A threaded insert can also add an additional $1,000-$2,000 to the cost of the tool, depending on the complexity of the part ejection method. By contrast, Fortify’s 3D-printed threaded insert added minimal time and cost to the project.
The two-piece assembly of the inserts streamlined production by minimizing both the material needed to 3D print molds for each geometry and the required force for finished part ejection, reducing the required force enough that the team could remove finished parts by hand.
T-splitters made with the 3D-printed mold tooling met Ventilator Project’s requirements for fit, form and function while enabling validation testing using injection-molded polypropylene — all while saving thousands of dollars and weeks of lead time compared to traditional aluminum tooling.
In the end, where traditional aluminum tooling would have cost $4,000 and taken between three to six weeks of lead time, the 3D-printed molds produced by Fortify only cost $700 with a lead time of seven days. With these benefits, Ventilator Project was able to select one design with maximum confidence — partnering again with Fortify to run those inserts on the company’s in-house press.
Related Content
ActivArmor Casts and Splints Are Shifting to Point-of-Care 3D Printing
ActivArmor offers individualized, 3D printed casts and splints for various diagnoses. The company is in the process of shifting to point-of-care printing and aims to promote positive healing outcomes and improved hygienics with customized support devices.
Read MoreDurable, Waterproof 3D Printed Casts: The Cool Parts Show #58
Recovering from an injury with an ActivArmor cast means that patients can exercise, bathe and live life while they heal. We get a firsthand look at the solution in this episode of The Cool Parts Show.
Read MoreUltra-Complex 3D Printed Scaffolds Enable Cell Growth: The Cool Parts Show #70
Perhaps the ultimate surface-area challenge is in bioengineering: creating structures that can grow sufficient cells within a compact volume to be effective for leading-edge medical treatments. The Southwest Research Institute develops bioreactor scaffolds that could only be made using 3D printing.
Read MoreMicro Robot Gripper 3D Printed All at Once, No Assembly Required: The Cool Parts Show #59
Fine control over laser powder bed fusion achieves precise spacing between adjoining moving surfaces. The Cool Parts Show looks at micro 3D printing of metal for moving components made in one piece.
Read MoreRead Next
Magnetic 3D Printing with Fiber Reinforcement Fills Tooling Gap
Fortify’s Digital Composite Manufacturing (DCM) platform pairs high-performance resins with fiber reinforcement that can be controlled at the voxel level. The process promises a faster route to durable injection mold tooling.
Read MoreInjection Mold or 3D Print? How Resolution Medical Pivots Production
Minnesota manufacturer Resolution Medical is finding opportunities for additive manufacturing via Carbon 3D printers as an alternative to injection molding for production.
Read MoreWhere 3D Printing Makes Sense for Micro Medical Devices
A contract manufacturer uses stereolithography to produce high-quality medical devices on a micro-scale for prototyping and end use.
Read More




















