3D Systems’ VSP Connect Streamlines Preoperative Planning for Better Patient Outcomes
Automated workflows powered by Enhatch drive efficiencies to deliver patient-specific devices, instrumentation at scale, while the portal improves visibility to facilitate better communication, traceability and compliance.
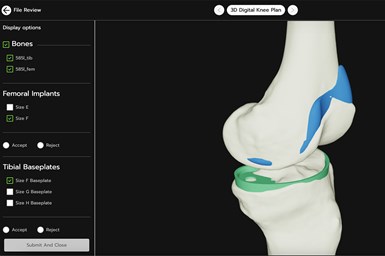
Surgeons can view patient-specific 3D models and comment on implant and guide placement at their convenience using VSP Connect to help streamline preoperative planning. Photo Credit: 3D Systems
3D Systems’ VSP Connect is a centralized, cloud-based surgical planning portal that integrates automated workflows and artificial intelligence (AI). As part of the company’s VSP surgical planning solutions, VSP Connect empowers device manufacturers and surgeons with real-time patient case visualization and improved collaboration capabilities.
VSP Connect is powered by Enhatch, with whom the company entered a partnership in 2022 to scale personalized medical device delivery. The solution incorporates Enhatch’s AI and automation technologies with 3D Systems’ FDA-cleared workflows for patient-specific solutions, including advanced software, expert planning services, personalized implant and instrument design, and industry-leading 3D printers and manufacturing processes. This combination is said to improve visibility for health care systems and medical device manufacturers, and streamlines the preoperative planning process — helping make personalized procedures more efficient while delivering improved outcomes.
“VSP Connect is the missing link in surgical planning, bridging the gap between patient care and cutting-edge technology,” says Michael Phipps, Enhatch CTO/president. “With Enhatch's advanced AI and automation capabilities, the portal gives surgeons the opportunity to reduce planning times and perform more patient-specific surgeries.”
3D Systems’ VSP surgical planning solutions are said to combine best-in-class digital workflows with the industry’s broadest additive manufacturing (AM) portfolio of printers and materials to deliver comprehensive patient-matched solutions. VSP Connect enhances these capabilities through automated workflows that strengthen communication between all stakeholders (such as device representatives, case managers, patient-specific device designers and surgeons), while facilitating compliance with industry regulations and internal accuracy protocols.
With the help of AI, VSP Connect offers pre-populated designs that are tailored to both individual surgeon preferences as well as to standard types of products. The secure, cloud-based portal aggregates disparate processes to provide a single intuitive interface enabling 24/7 access to the status of a case and the ability to send notes or alerts. This seamless, end-to-end experience streamlines the preoperative planning process — from surgical planning to the production and delivery of patient-specific implants and instruments — resulting in reduced procedure times and improved patient outcomes.
“3D Systems is on a mission to transform health care through the use of additive manufacturing to make patient care easier and smarter,” says Benjamin Johnson, vice president, portfolio and regulatory, health care, 3D Systems. “With VSP Connect, we are providing access to the health care industry’s most complete additive manufacturing ecosystem. Combined with Enhatch technology, our unified approach makes it easier to deliver patient-specific implants and instrumentation in a more efficient, cost-effective manner. It’s part of our ongoing commitment to innovation and helps ensure our customers are at the forefront of medical device development and healthcare delivery.”
3D Systems has worked with surgeons for more than a decade to plan more than 150,000 patient-specific cases, and manufacture more than two million implants and instruments for 100+ CE-marked and FDA-cleared devices from its FDA-registered, ISO 13485-certified facilities in Littleton, Colorado, and Leuven, Belgium.
- Learn about 3D Systems’s Regenerative Tissue Program for Surgical Reconstruction. The first product under development targets patient-specific regenerative breast tissue.
- Read about 3D Systems’ VSP Bolus for optimizing radiotherapy targeting. The system is designed to enable the creation of a more personalized bolus made from a soft material that contours to the patient’s anatomy, enabling a more efficacious treatment and a more comfortable experience.
Related Content
ActivArmor Casts and Splints Are Shifting to Point-of-Care 3D Printing
ActivArmor offers individualized, 3D printed casts and splints for various diagnoses. The company is in the process of shifting to point-of-care printing and aims to promote positive healing outcomes and improved hygienics with customized support devices.
Read MoreUnderstanding PEKK and PEEK for 3D Printing: The Cool Parts Show Bonus
Both materials offer properties desirable for medical implants, among other applications. In this bonus episode, hear more from Oxford Performance Materials and Curiteva about how these companies are applying PEKK and PEEK, respectively.
Read MoreOvercoming Challenges with 3D Printing Nitinol (and Other Oxygen-Sensitive Alloys) Through Atmospheric Control
3D printed nitinol has potential applications in dental, medical and more but oxygen pickup can make this material challenging to process. Linde shares how atmospheric monitoring and the use of special gas mixtures can help maintain the correct atmosphere for printing this shape alloy and other metals.
Read MoreMicro Robot Gripper 3D Printed All at Once, No Assembly Required: The Cool Parts Show #59
Fine control over laser powder bed fusion achieves precise spacing between adjoining moving surfaces. The Cool Parts Show looks at micro 3D printing of metal for moving components made in one piece.
Read MoreRead Next
Alquist 3D Looks Toward a Carbon-Sequestering Future with 3D Printed Infrastructure
The Colorado startup aims to reduce the carbon footprint of new buildings, homes and city infrastructure with robotic 3D printing and a specialized geopolymer material.
Read MoreBike Manufacturer Uses Additive Manufacturing to Create Lighter, More Complex, Customized Parts
Titanium bike frame manufacturer Hanglun Technology mixes precision casting with 3D printing to create bikes that offer increased speed and reduced turbulence during long-distance rides, offering a smoother, faster and more efficient cycling experience.
Read More3D Printed Polymer EOAT Increases Safety of Cobots
Contract manufacturer Anubis 3D applies polymer 3D printing processes to manufacture cobot tooling that is lightweight, smooth and safer for human interaction.
Read More