Lithoz, Himed Partner for Research on 3D-Printable Medical-Grade Bioceramics
This collaboration is designed to help grow the range of biocompatible materials suitable for a future that includes highly customized, patient-specific medical solutions that can be printed on demand.
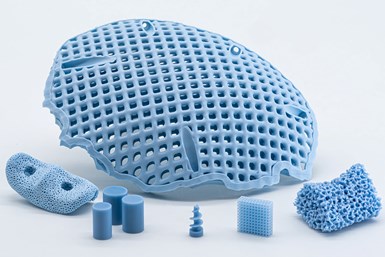
LithaBone HA 480 is a greatly improved human bone-like ceramic material which can be used to create distinctly blue complex parts. Photo Credit: Lithoz
Lithoz, a provider of ceramic 3D printing, and New York-based biomaterials manufacturer Himed have signed strategic research partnership agreement to further accelerate the development of medical-grade bioceramics. Focusing on implants using biocompatible calcium phosphates, the cooperation is designed to better meet the strongly increasing demand for innovative 3D-printable bioceramic feedstocks.
As part of the joint effort, Himed has acquired a Lithoz CeraFab S65 Medical Printer to explore novel integrations of its leading calcium phosphate biomaterials with Lithoz’s proprietary ceramic binder LithaBone HA 480.
After searching for a strategic partner in the United States to better access the potential of top-level surgery specialists there, this partnership forms the next logical step in the large-scale commercialization of this very specific 3D printing product. The partners say their collaboration will also help form an intercontinental pool of top researchers driving 3D-printable bioceramics to the next level. Partnering with Himed enables Lithoz to explore the potentials of other CaP phases within the sophisticated biomimetic forms its printers can make.
Thanks to its properties (such as excellent biocompatibility and osteoconductivity), the tricalcium phosphate or hydroxy apatite-based LithaBone medical ceramics have proven to be an attractive alternative to metals used in human surgery. Lithoz has also received numerous innovation study requests involving these bioceramics, as the precision and design flexibility of Lithoz LCM technology offers potential for innovation when it comes to lattice structures and porostities achieving the desired ideal level of osteoconductivity.
First-phase research will occur at Lithoz’s U.S. location in Troy, New York, this summer by a joint team of materials scientists. Himed will install a new Lithoz CeraFab S65 printer at their 25,000 ft.2 facility in Long Island early this fall, enabling the company to more rapidly experiment on-site and run analytical testing at its in-house laboratory.
The addition of a CeraFab S65 at Himed also broadens its R&D service offerings, adding rapid prototyping of different forms for clients who contract with Himed to conduct unique biomaterials research.
“Himed understands CaP optimization and how to scale it for a growing market. We’ve refined many calcium phosphates to strengthen their healing potential, but most of these were targeted toward surface coatings on traditionally manufactured titanium implants,” says Dana Barnard, Himed CEO. “Lithoz’s remarkable 3D printing technology allows a whole new direction for our products, in which we can use CaP to its greatest advantage — as a biomimetic material within the implant structure itself that can be replaced by a person’s own natural bone over time.”
Ultimately, both companies believe there is still much to discover about developing CaP materials to augment the performance of 3D-printed implantable forms. “This is definitely a big milestone for our partnership, and just a first starting point for a mutual beneficial collaboration for additive manufacturing of bone replacement,” says Dr. Johannes Homa, Lithoz CEO.
This strategic partnership represents a first step in growing the range of biocompatible materials suitable for a future that includes highly customized, patient-specific medical solutions that can be printed on demand. Over the last 30 years, calcium phosphates such as hydroxy apatite have gained widespread use in implantable devices, bone putties and grafting materials for their similarity to natural bone, and can aid the organic regrowth of hard tissue at the implantation site. Since 1991, Himed has collaborated with different medical implant manufacturers to develop and optimize various CaP powders and surface treatments for osseointegration. The partnership with Lithoz, however, enables new opportunities for Himed in the medical additive manufacturing market beyond bioactive surface treatments and postprocessing of implants.
- Learn more about Lithoz offering improved ceramic material for 3D-printed bone replacements. The company says the 3D printed, patient-specific parts made of hydroxyapatite are well suited for bone replacements in the medical and dental field.
- Read about Lithoz’s 3D printed ceramics serving as both bone graft and support. Bioceramics including tricalcium phosphate and zirconia have been used to replace and stabilize human bone in reconstructive surgeries. Now, 3D printing brings customization and new design opportunities to these medical devices.
Related Content
Bike Manufacturer Uses Additive Manufacturing to Create Lighter, More Complex, Customized Parts
Titanium bike frame manufacturer Hanglun Technology mixes precision casting with 3D printing to create bikes that offer increased speed and reduced turbulence during long-distance rides, offering a smoother, faster and more efficient cycling experience.
Read More8 Cool Parts From Formnext 2024: The Cool Parts Show #78
End-use parts found at Formnext this year address various aspects of additive's advance, notably AM winning on cost against established processes.
Read More3D Printed Lattices Replace Foam for Customized Helmet Padding: The Cool Parts Show #62
“Digital materials” resulting from engineered flexible polymer structures made through additive manufacturing are tunable to the application and can be tailored to the head of the wearer.
Read MoreActivArmor Casts and Splints Are Shifting to Point-of-Care 3D Printing
ActivArmor offers individualized, 3D printed casts and splints for various diagnoses. The company is in the process of shifting to point-of-care printing and aims to promote positive healing outcomes and improved hygienics with customized support devices.
Read MoreRead Next
Alquist 3D Looks Toward a Carbon-Sequestering Future with 3D Printed Infrastructure
The Colorado startup aims to reduce the carbon footprint of new buildings, homes and city infrastructure with robotic 3D printing and a specialized geopolymer material.
Read More3D Printed Polymer EOAT Increases Safety of Cobots
Contract manufacturer Anubis 3D applies polymer 3D printing processes to manufacture cobot tooling that is lightweight, smooth and safer for human interaction.
Read MoreCrushable Lattices: The Lightweight Structures That Will Protect an Interplanetary Payload
NASA uses laser powder bed fusion plus chemical etching to create the lattice forms engineered to keep Mars rocks safe during a crash landing on Earth.
Read More