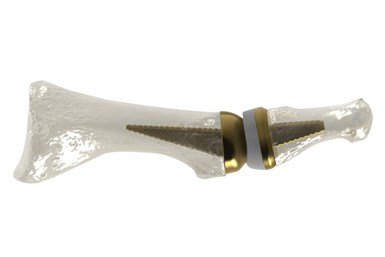
AddUp and Anatomic Implants Collaborate on FDA Submission for 3D Printed Toe Joint Replacement
Anatomic Implants has chosen to use AddUp’s FormUp 350 Powder Bed Fusion machine to qualify the implant for submission to the FDA. The FormUp 350 is able to produce varying complex geometries with fine detailed lattice structures, which are well suited for implantable medical devices.
Share
Read Next
AddUp and Anatomic Implants, a medical device startup based in Washington, D.C., are working together on a 510(k) submission for what they say is the world’s first 3D printed toe joint replacement. Anatomic Implants says it is the first medical device startup company to patent and develop a 1st metatarsophalangeal (MTP) joint replacement that nearly perfectly replicates the human anatomy by leveraging titanium 3D printing technology.
The MTP toe joint is located at the base of the big toe and is one of the three main points used for balance. It is often the first joint in the foot to develop osteoarthritis. According to the company, the global market for the 1st MTP joint reconstruction is more than $500 million each year, while the market is underserved with very few products — none of which are anatomic or have the potential to support bone-in growth as well as the Anatomic Great Toe Joint.
Through the use of additive manufacturing (AM), a porous structure can be integrated into the design to promote osseointegration. Osseointegration gives implants a much higher chance of bonding to the bone, which can reduce the chances of the implant being rejected by the body. This can also lead to better patient outcomes after surgery.
To bring its product to market, the company has chosen AddUp’s FormUp 350 Powder Bed Fusion (PBF) machine to qualify the implant for submission to the FDA. The FormUp 350 is able to produce varying complex geometries with fine detailed lattice structures, which are well suited for implantable medical devices. The FDA’s Center for Devices and Radiological Health (CDRH) and the Center for Drug Evaluation and Research (CDER) has approved many 3D printed class II medical devices through the 510(k) pathway since the mid-2000s.
“With 1st MTP joint replacement being a largely underserved market, and medical device companies building lattice structures into implantables since the mid 2000s, Dr. Nutter and I sought out to make a more anatomic design by leveraging the latest technologies adopted by the industry and FDA,” says David Nutter, Anatomic Implants president. “We were excited to partner with AddUp to achieve 510(k) clearance after learning about their proprietary 3D printing technology and seeing how it could benefit the development of the Anatomic Great Toe Joint. We look forward to leveraging the AddUp team and their expertise to validate the world’s first 3D printed toe joint replacement on their FormUp 350.”
The 510(k) clearance process involves a comprehensive review of safety and performance data for the implant to determine if it is substantially equivalent to an implant that is already on the market. Several tests have already been completed and the company anticipates 510(k) clearance in late Q3 2024. Anatomic Implants has been working on the Anatomic Great Toe Joint since its inception late 2016 and has already secured design patents in the U.S., Canada and throughout Europe — which represents the majority of the global 1st MTP joint reconstruction market.
AddUp has a lot of experience in the medical industry with global OEMs relying on the FormUp 350 for serial production of their medical implants. The company’s North American subsidiary, The AddUp Solution Center, is located in Cincinnati, Ohio, and is ISO13485 certified. It has experience partnering with a variety of medical customers and supporting their path to FDA clearance.
“AddUp is committed to supporting the development of cutting-edge solutions for the medical market,” says Nick Estock, AddUp Deputy CEO. “Our team at the AddUp Solution Center has the expertise on FDA regulations and qualification protocols to provide a proactive approach to regulatory compliance essential for a successful 510(k) submission. We are excited to be supporting Anatomic Implants through this process to bring the first additively manufactured toe joint replacement to market.”
Anatomic Implants was founded by Johns Hopkins Surgeon Scott W. Nutter, DPM, and David Nutter. Both share a passion for innovation, entrepreneurship and helping people thrive through enabling them to walk better. Dr. Nutter served as chief of podiatry at Washington Adventist Hospital (now the White Oak Medical Center) for several years. He has performed many 1st MTP joint replacements, and has used every total joint replacement system that has been developed for the 1st MPJ. It was through his experience as a double board-certified physician that he had a desire to create a better product to support patient needs.
David Nutter is an entrepreneur with a track record of successful startups across a variety of industries. David contracted Jalex Medical, a medical device design and regulatory affairs firm, in 2017 to jumpstart the development of the Anatomic Great Toe Joint.
Related Content
How Machining Makes AM Successful for Innovative 3D Manufacturing
Connections between metal 3D printing and CNC machining serve the Indiana manufacturer in many ways. One connection is customer conversations that resemble a machining job shop. Here is a look at a small company that has advanced quickly to become a thriving additive manufacturing part producer.
Read More3D Printed Lattice for Mars Sample Return Crash Landing: The Cool Parts Show Bonus
NASA Jet Propulsion Laboratory employs laser powder bed fusion additive manufacturing plus chemical etching to create strong, lightweight lattice structures optimized to protect rock samples from Mars during their violent arrival on earth.
Read MoreNew Zeda Additive Manufacturing Factory in Ohio Will Serve Medical, Military and Aerospace Production
Site providing laser powder bed fusion as well as machining and other postprocessing will open in late 2023, and will employ over 100. Chief technology officer Greg Morris sees economic and personnel advantages of serving different markets from a single AM facility.
Read More3D Printed Spacer Grids for Nuclear Power: The Cool Parts Show #79
Westinghouse Electric Company is exploring laser powder bed fusion as a means of manufacturing spacer grids, critical parts of the fuel rod assemblies used in pressurized water reactors.
Read MoreRead Next
Profilometry-Based Indentation Plastometry (PIP) as an Alternative to Standard Tensile Testing
UK-based Plastometrex offers a benchtop testing device utilizing PIP to quickly and easily analyze the yield strength, tensile strength and uniform elongation of samples and even printed parts. The solution is particularly useful for additive manufacturing.
Read MoreBike Manufacturer Uses Additive Manufacturing to Create Lighter, More Complex, Customized Parts
Titanium bike frame manufacturer Hanglun Technology mixes precision casting with 3D printing to create bikes that offer increased speed and reduced turbulence during long-distance rides, offering a smoother, faster and more efficient cycling experience.
Read MoreAlquist 3D Looks Toward a Carbon-Sequestering Future with 3D Printed Infrastructure
The Colorado startup aims to reduce the carbon footprint of new buildings, homes and city infrastructure with robotic 3D printing and a specialized geopolymer material.
Read More