Aerospace Manufacturer Pivots to 3D Print Masks for COVID-19 First Responders
Rapid Application Group is finalizing a 3D printed mask to protect first responders and medical staff who may be exposed to coronavirus. The nylon mask uses a replaceable N-95 or HEPA filter.
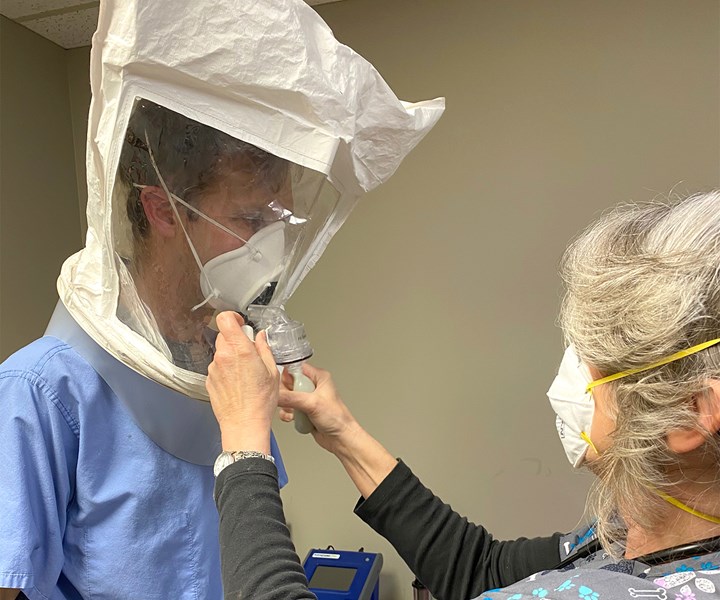
Rapid Application Group’s 3D printed RAG mask with a removable filter has passed fit tests at a local clinic, and is on its way to full FDA approval for clinical use. Photos: Rapid Application Group
“We’ve seen all of these companies and home makers jump on the bandwagon of printing a mask,” says Terry Hill, owner and CEO of Rapid Application Group (RAG). “We haven’t slept in the last four or five days because we want to make sure this is correct — that the material science is good, and the design is good.”

Jason Dickman, co-founder and COO, shows the 3D printed mask (pictured here without the filter in place).
Hill and his team at RAG have spent those sleepless days in a whirlwind of product development to create a safe and practical 3D printed mask to protect against coronavirus. The device is intended to be used as personal protective equipment (PPE) by first responders and medical support staff. I spoke with Hill and Jason Dickman, COO, on Thursday morning, and later that afternoon the mask passed a final fit test at local clinic in Tulsa, Oklahoma.
The RAG mask is based on previous designs and consists of two plastic 3D printed components that hold an N-95 or HEPA filter, which can be cut from an existing mask or larger piece of material. A food-grade silicone tube in the lip of the mask creates a comfortable seal for the wearer. The reusable mask can be disassembled and cleaned between uses, and the filter replaced as needed. One N-95 mask could provide 4 to 6 filters for the RAG mask.
Notably, the mask is designed not necessarily to replace N-95 masks, but to free up more of them by providing a protective alternative for other healthcare professionals.
“Field-level or nonsurgical PPE can save lives right now, and maybe put some of those other N-95 masks and other surgically approved items into the specific areas where they are needed,” Dickman says.
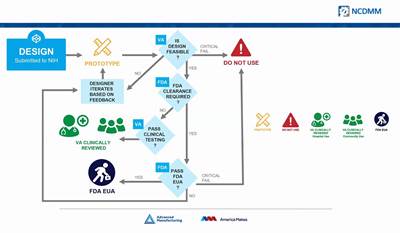
The idea to print PPE was at first an independent initiative on the part of Rapid Application Group, but the company is a member of America Makes and has worked with the VA and FDA and other partners on the RAG mask. While the emphasis currently is on field use, the company is in the process of seeking full FDA approval for use in a clinical setting. (At least one similar design submitted through the NIH 3D Print Exchange has been approved as a suitable stop-gap measure for clinical use.)
While the company intends to share the design once fully documented, Hill and Dickman both caution against 3D printing masks without due diligence. “We spent a lot of time planning to make sure we get this right,” Dickman says. “These are medical devices so you have to be careful; you can’t make any assumptions with this kind of scenario.”
RAG, which typically serves the aerospace and defense industries, is now preparing to ramp up to print production quantities of its masks using selective laser sintering (SLS) 3D printers from 3D Systems and EOS. The printed components are made with DuraForm PA or nylon PA12; both are USP Class VI materials suitable for medical devices that can be sterilized and are autoclavable. The printed parts are unpacked and cleared of powder, and then cleaned with a dipping procedure in the company’s mechanical testing lab which has been converted into a cleaning station for this purpose.
“We’re not a full medical company, so we can’t guarantee 100% sterilization,” Hill says, “but we are trying to get them as clean as possible. That’s why we’re using the materials we are. As [the PPE] comes into the hospital, they can sterilize it.”
The SLS process requires no support structures and allows parts to be stacked in the build volume; Rapid Application Group estimates that it will be able to nest up to 120 masks in a single print. The company already has orders from a hospice clinic, the U.S. Army and a number of local healthcare providers.
Along with masks, Rapid Application Group is also 3D printing face shield visors that can be equipped with any transparent plastic with holes made with a standard three-hole punch. The design features a locking mechanism at the temples to easily attach a shoe string or other strap.
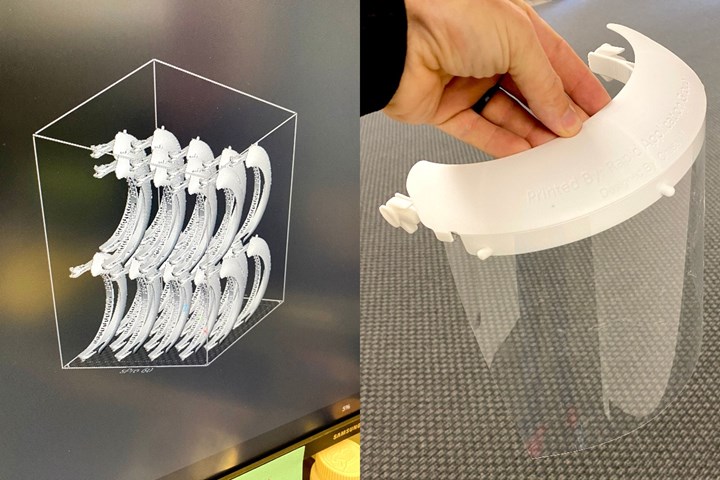
Rapid Application Group refers to this as the “Chase shield” after the company’s designer who created it.
Related Content
FDA-Approved Spine Implant Made with PEEK: The Cool Parts Show #63
Curiteva now manufactures these cervical spine implants using an unusual 3D printing method: fused strand deposition. Learn how the process works and why it’s a good pairing with PEEK in this episode of The Cool Parts Show.
Read MoreCranial Implant 3D Printed From Hydroxyapatite Ceramic: The Cool Parts Show #76
Cranial implants are typically made from titanium or PEEK; in this episode of The Cool Parts Show, we look at how implants made from a bioceramic can improve osseointegration and healing.
Read MoreActivArmor Casts and Splints Are Shifting to Point-of-Care 3D Printing
ActivArmor offers individualized, 3D printed casts and splints for various diagnoses. The company is in the process of shifting to point-of-care printing and aims to promote positive healing outcomes and improved hygienics with customized support devices.
Read MoreIce 3D Printing of Sacrificial Structures as Small as Blood Vessels
Using water for sacrificial tooling, Carnegie Mellon researchers have created a microscale method for 3D printing intricate structures small enough to create vasculature in artificial tissue. The biomedical research potentially has implications for other microscale and microfluidics applications.
Read MoreRead Next
3D Printing and Coronavirus Check-In - Week of 3/30/2020
In this week's discussion, editors Peter Zelinski and Stephanie Hendrixson discuss face shield visors and masks; ventilator components; and new movement from the FDA on 3D printed devices.
Read More3D Printing and Coronavirus: U.S. Additive Manufacturers Share Their Experiences
The COVID-19 outbreak has brought both setbacks and opportunities for American manufacturing. 3D printing companies share their stories.
Read MoreAmerica Makes Partners with FDA, NIH and VA to Coordinate Coronavirus-Related AM Efforts
"We're trying to be the matchmaker" between additive manufacturers, hospitals and designers, says John Wilczynski, executive director of America Makes.
Read More
.jpg;width=70;height=70;mode=crop)