America Makes Partners with FDA, NIH and VA to Coordinate Coronavirus-Related AM Efforts
"We're trying to be the matchmaker" between additive manufacturers, hospitals and designers, says John Wilczynski, executive director of America Makes.
America Makes is stepping up to organize an additive manufacturing (AM) response to medical equipment shortages as a result of the coronavirus pandemic. In a webinar on Monday, March 30, Executive Director John Wilcyznski described the organization’s efforts to serve as a central source of information by compiling information from three types of stakeholders: healthcare providers, designers and manufacturers. America Makes has partnered with Veterans Affairs (VA), the National Institute of Health (NIH) and the Food and Drug Administration (FDA) in this effort.
As we’ve reported in recent weeks, the additive manufacturing community has been willing and eager to help make PPE and other necessary equipment. But, information about which hospitals need these items, what they need, and where to find approved designs has been difficult to locate. “We’re trying to be the collection point for information,” Wilcyznski says.
The organization’s COVID-19 landing page now has entryways for each type of stakeholder. Hospitals and other healthcare providers can enter their needs; designers can provide open source files for needed items; and manufacturers can input their 3D printing capabilities. When a need is specified, America Makes works to match the healthcare client with manufacturers who have the needed capability. As Wilcyznski explained, the site is not a marketplace, but a repository that can help be a matchmaker between parties. For the time being America Makes will attempt to match medical needs with manufacturers who can meet them, and leave it up to the medical client to initiate outreach to a chosen manufacturer. Eventually, medical users will be able to query the data and receive a list of results for manufacturers who can help. Business negotiations will happen off-platform between the concerned parties.
Perhaps the most significant development through this project, however, is a clear decision-making pathway for 3D printed medical devices, something that has been lacking thus far. Designers entering through the America Makes landing page will be led to the NIH 3D Print Exchange (a collection of open-source biomedical designs) to upload their files and other information. Designs entered through this system will be assessed first by the VA for viability, perhaps with design changes. Items that are approved at this stage and do not require FDA clearance can then pass into clinical testing with the VA. For devices that do require clearance, the FDA will get involved with the approval process or emergency use application (EUA) clearance. All designs will be available through the NIH Exchange with their appropriate label.
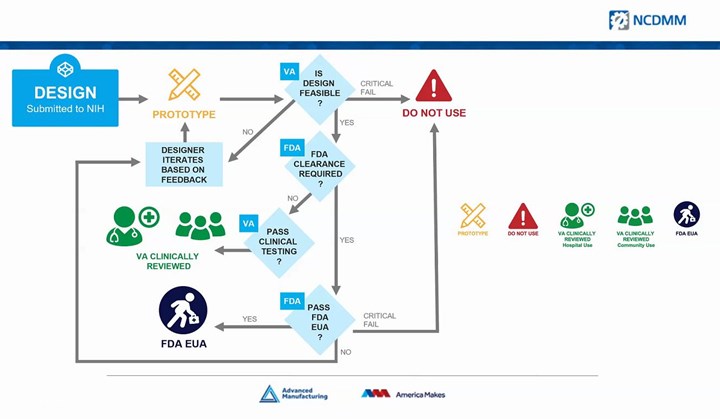
America Makes is providing a clear pathway to FDA clearance for viable 3D printed medical devices that require it. Screenshot: America Makes webinar
The goal is not just to expedite FDA clearance, but to help direct and focus industry efforts. For instance, the VA is currently evaluating a number of open source face shield designs and working to narrow the field the best possible equipment. Devices submitted through this system can be designed for use in hospitals or healthcare facilities, or a community setting such as grocery stores.
Related Content
-
3D Printed Spine Implants Made From PEEK Now in Production
Medical device manufacturer Curiteva is producing two families of spinal implants using a proprietary process for 3D printing porous polyether ether ketone (PEEK).
-
FDA-Approved Spine Implant Made with PEEK: The Cool Parts Show #63
Curiteva now manufactures these cervical spine implants using an unusual 3D printing method: fused strand deposition. Learn how the process works and why it’s a good pairing with PEEK in this episode of The Cool Parts Show.
-
Q&A With Align EVP: Why the Invisalign Manufacturer Acquired Cubicure, and the Future of Personalized Orthodontics
Align Technology produces nearly 1 million unique aligner parts per day. Its acquisition of technology supplier Cubicure in January supports demand for 3D printed tooling and direct printed orthodontic devices at mass scale.

.jpg;width=70;height=70;mode=crop)