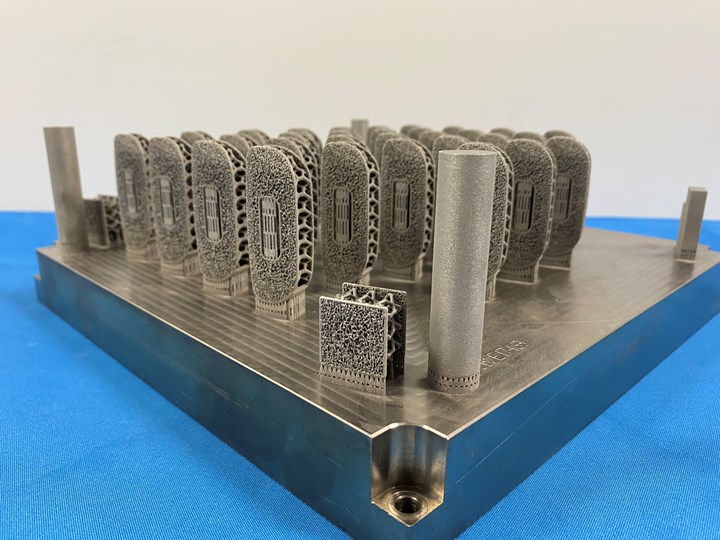
RMS bought its first 3D Systems DMP 350 Flex laser powder bed fusion 3D printer in 2016. Now the company has its own additive division with 30 machines that run almost continuously to produce as many as 20,000 3D printed implants per month. Photo provided by RMS Machining.
With 30 laser powder bed fusion machines running almost continuously to produce up to 20,000 implants per month, RMS Machining is unquestionably succeeding at production additive manufacturing. This additive capability builds off of RMS’s subtractive manufacturing operation, which has more than 650 machine tools ranging from five-axis milling to Swiss-type and multi-spindle lathes. The company has 1,100 employees and ships 3.5 million components per month from its 350,000 square foot facility in Coon Rapids, Minnesota. This makes RMS the largest company within Cretex Medical, a family of companies that provides medical manufacturing services including machining and additive manufacturing, plastic injection molding, stamping and sterile packaging for all types of medical devices, from implants to instrumentation cases and trays.
While RMS’s subtractive manufacturing operation machines a range of orthopedic implants and cardiac components, the majority (80%) of the parts made in the AM unit are spinal implants. All 3D printed implants are made of Ti64, the only material RMS currently uses in its AM unit, and all leverage the advantages of AM to produce bone ingrowth surfaces.
RMS started looking into additive manufacturing around 2013. Based on both direct customer feedback and information found in FDA filings by medical device manufacturers, the company realized that many of these companies were researching AM platforms, specifically laser powder bed fusion platforms from 3D Systems. RMS used this information to make a case to its parent company to invest in additive manufacturing and installed its first 3D Systems DMP ProX 320 in early 2016.
For the next year, RMS didn’t advertise this capability to its customers and focused on learning how to use the system from the ground up. “We needed to learn the basics of operating additive machinery, including software and powder management,” says Troy Olson, director of operations for RMS’s additive division. To help with this process, the company brought Ryan Kircher, a former 3D Systems employee with experience in 3D printed medical devices, on board as a consultant. He recently re-joined the company, this time as a full-time senior additive manufacturing engineer.
Roughly 80% of the RMS additive division’s work is producing spinal implants. All 3D printed implants are made of Ti64 and include bone ingrowth surfaces.
By 2018, the additive manufacturing division was becoming profitable. Since then, the additive business has grown on average at a double-digit rate year-over-year. Olson notes that this growth is all new business — it is incremental sales growth and is not taking work away from the company’s subtractive division.
In order to support this level of production, the additive division has grown to include not only the 30 printers, but also a dedicated machine shop to perform finish machining on the additively manufactured parts.
As RMS established and grew its additive division, it had to figure out how to manage additive production. The additive team at RMS began by applying their accumulated knowledge of best practices from their successful subtractive machining operations. As the company has learned more and grown its capacity, it has discovered where a subtractive mindset applies in additive manufacturing, and where it does not.
Standardized Machines, Nonstandard Jobs
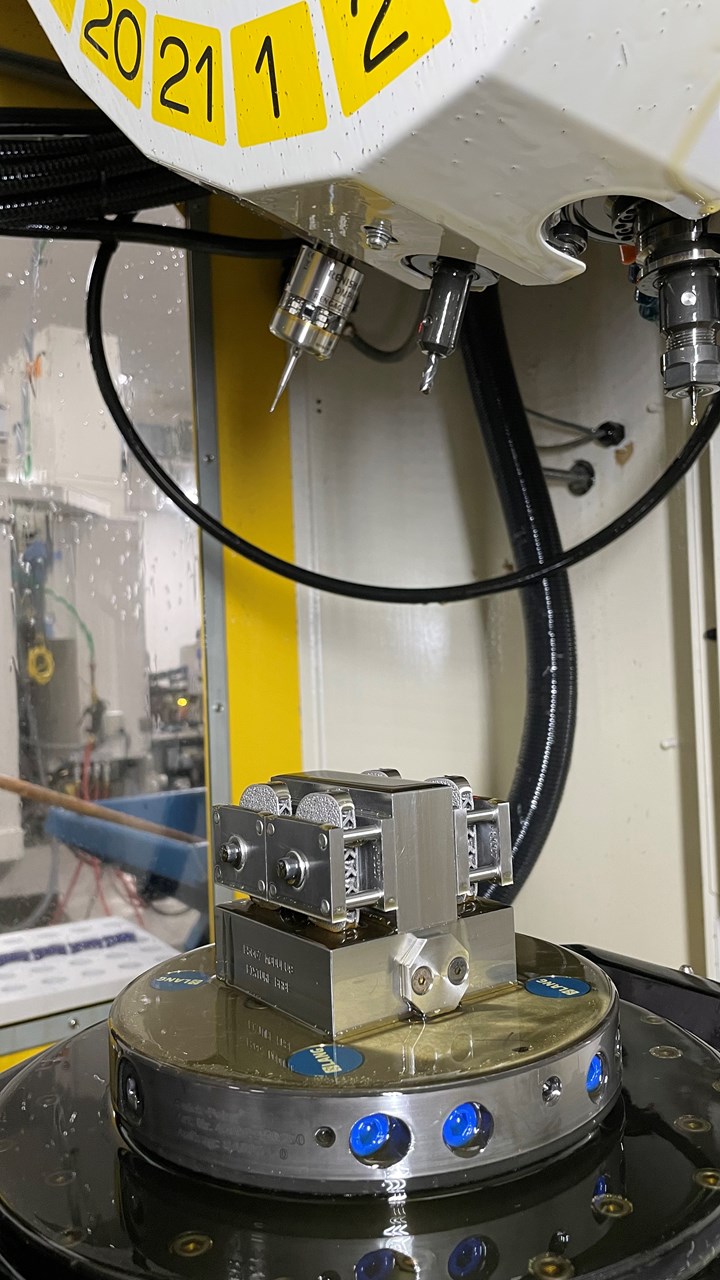
One lesson that has carried over from subtractive to additive is the standardization of machines to achieve maximum flexibility. RMS’s shop floor is filled with groups of identical machine tools, and the additive division is no different — all 30 of its direct metal printers are from 3D Systems, either the DMP ProX 320 or DMP Flex 350 machines. Olson notes that there are some slight variations in the machines because some of them were purchased at different times, but they all function in the same way because they have the same capabilities and software. As RMS adds new printers, it upgrades the older machines. “We usually buy them with every option you possibly can get on them because through the lifecycle of this printer, we don't know what it's going to have to produce,” Olson says. The same applies for the additive machine shop. All 12 of its machine tools are FANUC Robodrills. Even the tool carts for additive machine operators are standardized, each containing all of the tools needed to change over a machine.
The additive division also has its own dedicated machine shop equipped with 10 Robodrill machining centers staffed by specialized operators. Olson says that all implants produced by the additive division require finish machining.
In building its additive capacity, RMS has had to train its additive machine operators in-house, many of whom started off in the subtractive division. “If we're looking for a machinist, we put a sign on the corner, we have an open house and we have local machinists that are looking for opportunities come here,” Olson says. “When it comes to 3D printing, they're all home grown. There are no DMP operators that are going to answer the ad.” Training requires more than just learning how to run one type of machine. The employees that run RMS’s 3D printers are also responsible for powder management (handling and sieving the material, as well as the PPE required for these tasks), running the wire EDMs that separate the parts from the build plates, removing supports, performing quality control inspections, and performing preventive maintenance on the machines. According to Olson, this requires a shift from the mindset of machine operators in other areas of the shop. “We’re asking them to do multiple different things throughout the course of the day, not just man this printer,” he says.
Building Around the Build Plate
Additive manufacturing also requires a drastic shift in the customer’s mindset. If, for example, the subtractive division gets an order for 1,000 parts that run on a Swiss machine, it can manufacture the whole order in a single setup as one job. But if the additive division gets an order for 1,000 spinal implants, it has to break that order down into a build plate quantity. “Every build plate becomes its own job, so the economies of scale, as far as costing is concerned, are lost,” Olson says. The tooling and setups are not amortized across production as in subtractive manufacturing. “Traditional buyers think, well, if I give you an order for 10,000 implants, I should get a substantial cost reduction,” he says. “We really have to step them through the costing structure, saying, really, you're buying 100 at a time, even though the order is for 10,000, because that's all we can put on a build plate.”
RMS has developed a system for breaking down orders into build plate quantities. First, it needs to know which test specimens need to be included, so it can populate the plate around them. From there, the biggest consideration is time. This started when RMS only had one machine and one operator. In order to keep the machine running, the operator configured all of the plates so that the builds would end when he was in the shop. He would set it up so the machine would be finished before he arrived in the morning. He could then change it over and do the paperwork and peripheral operations on the parts.
Builds are generally 46 or 72 hours. Seventy-two hours is the time limit for builds according to RMS’s established risk mitigation protocols.
Since then, the shop has continued to configure its build plates around timing. Builds are generally 46 or 72 hours. Seventy-two hours is the time limit for builds according to protocols that RMS established to mitigate risk.
Timing also affects how RMS staffs its printers. For example, if all 30 printers run over the weekend and are ready to be changed over on Monday morning, the eight operators on that shift would not be able to get to all of them. To address this, the shop added a weekend shift, and staggers the end times of its builds so it can keep them running. Olson says that on any given night, all of the machines are running (with maybe one down for preventive maintenance), enabling efficiency rates of 95%.
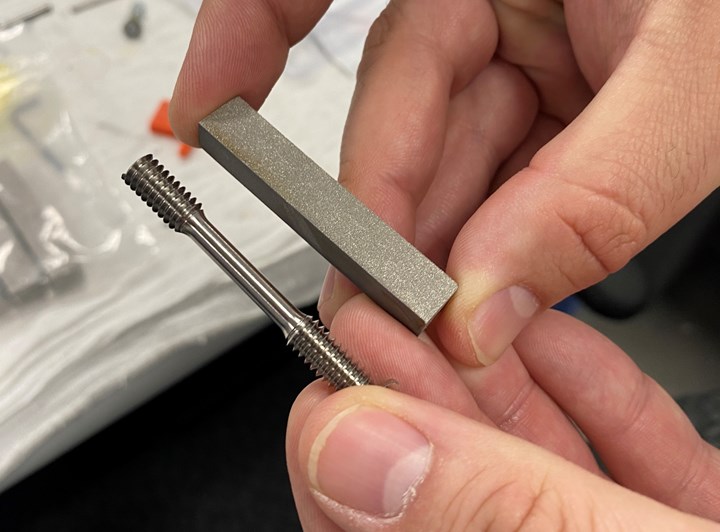
The build plate continues to affect production even after the parts are no longer attached to it. After being removed from the build plate, the parts are placed in a special tray and continue to travel together through downstream operations, including hot isostatic pressing (HIP), machining and cleaning. Machining is especially affected by the build plate lots — in traditional machining, an order of 1,000 parts needs only one setup. But if an order of 1,000 3D printed parts is split into 10 build plates, it will require 10 separate setups.
Even after the 3D printed parts are separated from the build plate, they continue to travel together as a lot through downstream operations including hot isostatic pressing and machining. Each lot requires its own setup in the machine shop.
Customer Collaboration
Designing an additive manufacturing production process is a collaboration between the customer and RMS’s engineering, quality and machining teams. According to Olson, the sooner RMS gets involved in the process, the better. “When we can get engaged with our customers early on, we'll actually design the part around workholding and datum structures for post machining, because that's really one of our core competencies,” he says.
This level of collaboration varies depending on when in the design process RMS gets involved and how much experience the customer has with additive manufacturing. It usually starts with a conversation through which RMS can find out more about the project, as well as the customer’s level of knowledge about additive and how they arrived at RMS. Some customers have no experience with AM and need feedback on part design. Others have test parts from a service bureau but haven’t considered how to produce the part at scale. Olson says a few customers even have their own AM technology and develop their own printing processes, which they can hand off to RMS to implement and scale for serial production.
RMS collaborates closely with its customers on many aspects of the production process, including the design phase. When the company can get involved this early, it can ensure that the part is designed around workholding, which is typically a challenge for machining 3D printed implants.
RMS also needs to work closely with its customers on developing a quality system. As the company has experience producing medical devices, it already understands good manufacturing practices for record-keeping and traceability. While there are guidance documents (such as ASTM F3001 – 14, which specifically covers Ti64 components made with laser powder bed fusion), Olson thinks that the industry is still a few years away from consolidated standards. In the meantime, RMS has developed a rigorous baseline quality control system based on its experience and these guidance documents and customers can make adjustments from there. RMS’s additive division has done business with more than 20 companies so far and has found that quality systems vary. “We work with a variety of different companies from small startups to leading medical device manufacturers, and their perspectives can be completely different,” Kircher notes.
When establishing a quality system, RMS often works with customers to develop methods for testing critical features. “We help our customers develop coupons, including witness and quality control coupons, that are more specific to their device than just a large, standard tensile bar,” Kircher says. These test coupons, in turn, affect the way additive jobs are routed. Where a subtractive job requires one router, AM jobs have multiple. Each test component has its own paperwork that gets separated at certain points in the production process before the components of a build come back together at the end.
Test specimens also add time and cost to builds, so RMS ensures they are absolutely necessary when including them. The shop has been compiling data on all of its builds and plans to use this to reevaluate the use of some coupons. For example, if it has never had a failed tensile test and it evaluates regular preventive maintenance with tensile tests, does it need to continue adding a tensile bar to every build plate? Test specimens add extra time and cost, so why include them if they are not adding value? Kircher says customers do not always think long-term about this. “The easiest thing to do is just put a bunch of coupons on the build plate during product development and test it all so that you can know you have good results, and you can launch your product,” he explains. “But then, you're stuck to that quality control plan for the next five or 10 years, where if you would have taken a little extra time and been intelligent about how you set up your quality control to begin with, you can save several hundred dollars each build and weeks of lead time in testing that doesn’t necessarily provide any value.”
Adding to Additive Capacity
In addition to updating its quality standards, the RMS additive division has more changes planned. It recently built a lab that is in the final stages of approval and will allow the company to do its own material testing (including tensile, chemical, microstructural and compression) in-house. It is also looking at bringing other processes in-house, like HIP. In addition, the company is considering expanding into more large-joint implants, as well as new materials such as stainless steels, and additional technologies including dual-laser powder bed fusion and binder jetting.
However, as the additive division grows, the team will have to maintain its current success. “There's this fine line of staying focused and also branching out and doing new, interesting things, and you kind of want to keep them separate so you don't screw up what you're already really good at,” Kircher says.
Related Content
This Drone Bird with 3D Printed Parts Mimics a Peregrine Falcon: The Cool Parts Show #66
The Drone Bird Company has developed aircraft that mimic birds of prey to scare off problem birds. The drones feature 3D printed fuselages made by Parts on Demand from ALM materials.
Read More3D Printing with Plastic Pellets – What You Need to Know
A few 3D printers today are capable of working directly with resin pellets for feedstock. That brings extreme flexibility in material options, but also requires greater knowledge of how to best process any given resin. Here’s how FGF machine maker JuggerBot 3D addresses both the printing technology and the process know-how.
Read More8 Cool Parts From Formnext 2024: The Cool Parts Show #78
End-use parts found at Formnext this year address various aspects of additive's advance, notably AM winning on cost against established processes.
Read MoreVideo: 5" Diameter Navy Artillery Rounds Made Through Robot Directed Energy Deposition (DED) Instead of Forging
Big Metal Additive conceives additive manufacturing production factory making hundreds of Navy projectile housings per day.
Read MoreRead Next
How A Digital Manufacturing Workflow Is Making Orthoses, Prostheses More Accessible
Digitization of the workflow needed to create limb prostheses, orthoses, helmets and more means that these devices will become more readily available. Using 3D scanning and 3D printing, EastPoint Prosthetics and Orthotics and Additive America have established a way to produce these devices that both changes lives and makes business sense.
Read More3D Printing Brings Custom, Affordable Bone Prostheses to Thai Patients
Meticuly was founded to provide custom bone implants for local patients in Thailand and regional countries who otherwise would be dependent on standard, imported devices. The concept could be a model for medical treatment in other emerging economies.
Read MoreMore Than Meets the Eye to Cobra’s 3D Printed Putter
Cobra Golf drew attention in November 2020 with the launch of a limited-edition putter with metal 3D printed head. What this club says about product development, reshoring manufacturing and the future of consumer goods.
Read More