Additive Manufacturing Process Using Titanium Alloy Builds More Stable Spinal Implant
A spinal implant additively manufactured from a titanium alloy imitates cancellous bone in porosity and structure.
Conventionally manufactured bone implants are machined and then finished with a rough coating that helps anchor the implant and encourage the in-growth of cancellous bone, the spongy bone tissue found inside vertebrae and at the ends of large bones. Metal additive manufacturing goes beyond coating by offering the ability to build hollow matrices that extend below the surface. Implants with the texturing built-in are far more secure as the bone can grow into the device itself, eliminating the risk of detachment posed by surface coatings.
However, natural bone doesn't grow in a strict matrix or honeycomb structure; instead, it grows in a random arrangement of bridges and cavities. Medical manufacturer Stryker has created medical implants that more closely imitate the organic structure of cancellous bone, using its Tritanium in-growth technology. The strategy combines Tritanium, a proprietary titanium alloy, with the company's Laser Rapid Manufacturing technique, an additive manufacturing process, to create a completely randomized yet reproducible porous structure.
Tritanium is a highly porous titanium alloy designed specifically for bone in-growth and biological fixation. It has a mean porosity of 60 percent, with a mean pore diameter of 438 microns. Natural cancellous bone ranges from 50 to 90 percent porosity. Cancellous bone has a mean pore diameter of 1 mm, but pore diameters vary widely and randomly; thanks to the additive manufacturing technique, Tritanium imitates this with pore diameters ranging from 100 to 700 microns in a random architecture. The combination of material and process means that porous Tritanium has an elastic modulus that falls between spongy cancellous bone and more rigid cortical bone.
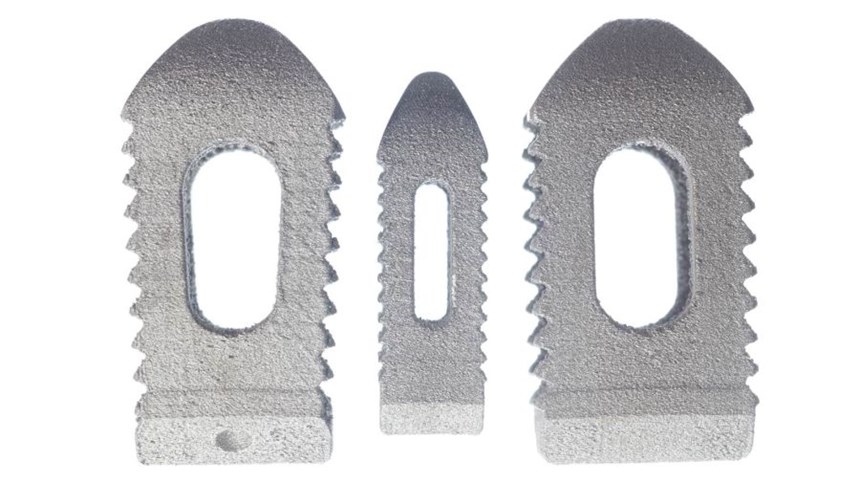
One example where this technology has made a difference is in Stryker Spine's PL cage, a posterior lumbar spinal implant, pictured above. This hollow, rectangular implant has a configuration of both solid and porous structures mimicking bone that are built simultaneously via laser-driven additive manufacturing. In addition to the cage's porous internal structure, its large central and lateral windows help to reduce overall stiffness, while allowing room for bone graft to be packed inside the cage. According to the company, the lateral windows also enable visualization via CT scan and X-ray.
Angled teeth with smooth leading edges increase the implant's surface area in contact with the bone and provide bidirectional stability. According to Stryker, the expulsion force for the implants has been shown to be 22 percent greater than the insertion force. The teeth also help to normalize load transmission and minimize subsidence (the sinking or settling of an implant). In testing, the Tritanium PL Cage demonstrated better resistance to subsidence than other commercially available posterior lumbar interbody cages constructed out of other materials, including those with a larger footprint.
The Tritanium PL cage is available in lengths ranging from 23 to 28 mm, widths from 9 to 11 mm, and heights from 7 to 14 mm. Learn more on this microsite dedicated to the spine cage.
Related Content
-
FDA-Approved Spine Implant Made with PEEK: The Cool Parts Show #63
Curiteva now manufactures these cervical spine implants using an unusual 3D printing method: fused strand deposition. Learn how the process works and why it’s a good pairing with PEEK in this episode of The Cool Parts Show.
-
Cranial Implant 3D Printed From Hydroxyapatite Ceramic: The Cool Parts Show #76
Cranial implants are typically made from titanium or PEEK; in this episode of The Cool Parts Show, we look at how implants made from a bioceramic can improve osseointegration and healing.
-
Durable, Waterproof 3D Printed Casts: The Cool Parts Show #58
Recovering from an injury with an ActivArmor cast means that patients can exercise, bathe and live life while they heal. We get a firsthand look at the solution in this episode of The Cool Parts Show.

.jpg;width=70;height=70;mode=crop)